-
PDF
- Split View
-
Views
-
Cite
Cite
Roger Bayston, Waheed Ashraf, Toni Smith, Triclosan resistance in methicillin-resistant Staphylococcus aureus expressed as small colony variants: a novel mode of evasion of susceptibility to antiseptics, Journal of Antimicrobial Chemotherapy, Volume 59, Issue 5, May 2007, Pages 848–853, https://doi.org/10.1093/jac/dkm031
Close - Share Icon Share
Abstract
Triclosan is in widespread use in domestic, commercial and healthcare settings and is used to reduce methicillin-resistant Staphylococcus aureus (MRSA) load in carriers. Triclosan resistance is uncommon, usually being due to mutation in fabI or overexpression of efflux pumps. This study investigated the ability of triclosan-containing silicone elastomer to kill MRSA adherent to its surface.
Silicone discs containing triclosan were prepared by a matrix-expansion method previously described. Discs were exposed to three strains of MRSA for 1 h for adhesion to take place. After incubation, discs were removed at intervals, sonicated and the sonicates analysed by chemiluminescence and viable counting. Survivors were found to consist of small colony variants (SCVs). These were then subjected to tests for known SCV characteristics and for susceptibility to triclosan.
Viable counts fell until 51 h, when they began to increase, due to SCV. Of the three SCV strains, two showed impaired coagulase production and all showed reduced deoxyribonuclease production. None was auxotrophic. MICs of triclosan in the SCV rose by between 8- and 67-fold.
Prolonged exposure of MRSA to triclosan-impregnated silicone, as in ‘antimicrobial’ plastics or catheters, resulted in the induction of SCV status and triclosan resistance. This has implications for industrial, medical and domestic use of polymers containing triclosan. SCVs are pathogenic and persistent. The widespread use of triclosan could lead to infection with MRSA SCVs, and new antimicrobials with physiological targets similar to that of triclosan might give rise to SCV resistance in clinical use.
Introduction
Plastics and other polymers containing biocides are a common feature of the domestic, commercial and healthcare environments, being found in lunchboxes, chopping boards, benchtops, knife handles and even children's toys.1 Their purpose is to reduce the popularly perceived risk of infection. The biocide most commonly used is triclosan, a biguanide antiseptic with a wide spectrum of activity. Surgical sutures containing triclosan are now available.2 It has also been used in ureteral stents3 and is widely used in handwashing4 and as a body wash to eradicate methicillin-resistant Staphylococcus aureus (MRSA) from carriers prior to surgery.5 Triclosan acts by inhibiting FabI, essential in bacterial fatty acid synthesis.6,7 Development of resistance following exposure is uncommon and could not be demonstrated following exposure of S. aureus to sub-MIC of triclosan,8 but spontaneous low-level resistance mutants have been reported in Escherichia coli and S. aureus, including MRSA.9 Previously reported resistance in these organisms has been thought to be due to either mutation in fabI leading to overexpression of the reductase10 or overexpression of efflux pumps.11 Extended or repeated exposure of S. aureus to polymers containing triclosan, as might be experienced in many of the settings where the biocide is used, has not previously been investigated. Here, we report development of resistance in MRSA during experimental exposure to determine the duration of antimicrobial activity of triclosan-impregnated silicone.
Materials and methods
Test bacteria
Three independent clinical isolates of EMRSA 15 were used, designated F1853, F1854 and F1855.
Triclosan-containing polymer
Triclosan was obtained from Ciba-Geigy (Macclesfield, UK). Silicone was chosen because of its high diffusion compatibility with triclosan and its clinical application as an implantable biomaterial. Medical grade silicone sheet 1 mm thick was cut into discs 4.8 mm in diameter. The method of impregnation, previously described,12 was briefly as follows: triclosan was dissolved in chloroform to give a final concentration of 0.2% (w/v). The discs were immersed in the chloroform for 1 h, after which they were removed and dried in a current of air for 1 h. They were then rinsed in ethanol to remove any surface accretions of triclosan and autoclaved at 121°C for 15 min. Silicone discs that had not been impregnated were included as controls. All assays were carried out on three discs, and quantification on each was carried out in triplicate.
Conditioning film
The discs were aseptically immersed in pooled human plasma (National Blood Authority, Sheffield, UK), with gentle rocking for 1 h at room temperature. They were then removed and gently rinsed in sterile water to remove surplus plasma.
Killing activity
An assay of the time taken to kill 100% of bacteria attached to the polymer surface (tK100) was carried out.13 Discs of the triclosan-containing silicone, with a plasma conditioning film, were immersed in a suspension of the test bacteria for 1 h for attachment to take place. They were then removed, gently rinsed in sterile water to remove non-attached bacteria and immersed in 2% tryptone soya broth (TSB; Oxoid Ltd, Basingstoke, UK), as this had previously been found by experiment to maintain viability but not to support proliferation. At intervals of 3, 6, 24, 27, 30, 48, 51, 54 and 72 h, these discs were removed from their suspensions and gently rinsed. In the case of the remaining discs, the TSB was changed at each time interval and they were returned to the incubator. The three removed discs were then immersed in 1 mL of sterile water and sonicated at 50 Hz (Ultrawave, Cardiff, UK) for 20 min to remove attached bacteria. The viable bacteria in the sonicates were enumerated in triplicate by chemiluminescence and plate counting.
Characterization of colonial variants
When it became clear that colonial variants were appearing after exposure of attached bacteria in the tK100 assay, tests were carried out on both wild-types and variants in an attempt to characterize these.
Clumping factor: the ability of bacterial suspensions to aggregate (clump) in the presence of human plasma on a microscope slide was determined.
Coagulase production: the time taken to produce a fibrin clot in human plasma at 37°C was determined. One millilitre of a suspension of two to three colonies of ‘small colony variant’ (SCV) (or one colony of the wild-type strains) in sterile water was added to 5 mL of either 10% or 50% human plasma in TSB. Tubes were inspected for clot formation every hour for 8 h, then after 18 h.
Deoxyribonuclease (DNAse) production: DNAse activity was determined semi-quantitatively on DNAse agar (Oxoid). After overnight incubation, zones of clearing after addition of 0.5 M hydrochloric acid were measured using callipers.
Haemolysin production: the production of haemolysin was sought around colonies on sheep blood agar plates (Oxoid) incubated in air at 37°C.
Auxotrophy: auxotrophy for haem, thiamine, coenzyme A and menadione was determined using sterile paper discs, each containing 1.5 µg of the above, placed on seeded RPMI agar (Oxoid) plates and incubated overnight. Auxotrophy would appear as increased colony size immediately around the discs.
Gentamicin susceptibility: susceptibility to gentamicin was determined using plate incorporation on Iso-Sensitest agar (Oxoid) at 37°C. Plates were read after 18 h of incubation, then re-read after a further 36 h of incubation due to the slow growth of the SCV.
Triclosan susceptibility: susceptibility to triclosan was determined by serial dilution. Doubling dilutions of triclosan in TSB were made in 96-well microtitre plates. Wild-types and variants isolated from the sonicates were suspended in sterile water and 10 µL of each was added to each well. After incubation, the highest dilution at which no visible growth (turbidity) was seen was taken as the MIC. Assays were carried out in triplicate.
Reversion to wild-type: the rate of reversion to parent type was determined by seeding suspensions of variant colonies onto sheep blood agar plates and, after overnight incubation at 37°C, estimating the proportion of wild-type colonies to variants. Attempts to produce revertants were also made by culturing in TSB.
ATP content: wild-types and SCVs of the test strains were incubated overnight in TSB with and without additional glucose to a total concentration of 0.5% (w/v). Cells were then harvested, washed in PBS and resuspended in PBS to A490 0.6, found by experiment to be equivalent for both wild-types and SCV to ∼1.5 × 107 cfu/mL. Aliquots of 100 µL were added in triplicate to chemiluminescence plates (Berthold Technologies, Bad Wildbad, Germany) and 100 µL of Bactolyse reagent (Cambrex Bioscience, Rockland, MA, USA) added. After 10 min, the tray was placed in a luminometer (MicrolumatPlus LB96v, Berthold Technologies) and the relative light units (RLUs) recorded using luciferase and ATP-monitoring reagent (Vialight, Lumitech, Nottingham, UK). RLU/ATP was determined from a standard curve using dilutions of ATP standard (Lumitech) and the amount of ATP per bacterial cell was calculated.
Results
Killing activity
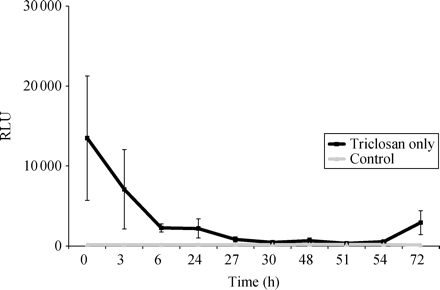
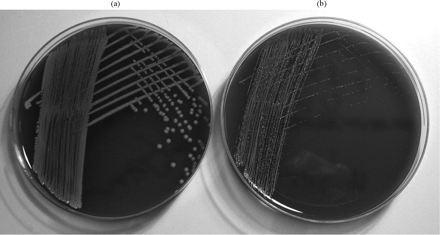
Chemiluminescence showed a progressive fall in numbers of viable attached bacteria until 51 h of exposure. The numbers then rose slightly, followed by a recovery to 22% of the inoculum at 72 h, at which point the assay was terminated (Figure 1). This was confirmed by plate cultures, which showed that the recovery was due almost entirely to the appearance of colonial variants (Figures 2 and 3).
The time taken to kill bacteria (F1854 MRSA) adherent to the triclosan-impregnated silicone, expressed as RLUs. All assays were done in triplicate. Note the fall in numbers of viable adhered bacteria, confirmed by plate cultures, until 51–54 h exposure, followed by an increase thereafter. Both F1853 and F1855 showed similar results.
(a) MRSA (F1854) recovered from the triclosan-impregnated silicone at 0 h (i.e. after 1 h adherence period). No SCVs are seen. (b) MRSA (F1854) after extended exposure to triclosan for 72 h. The culture now consists almost entirely of SCV. Again, both F1853 and F1855 gave similar results.
Magnification × 5 of SCV after 72 h of exposure of MRSA F1854 to triclosan, showing the difference in colony size and the lack of haemolysis in the SCV. A colour version of this figure is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Characterization of the variants
The results for (i)–(v) are shown in Table 1.
Comparison of the characteristics of wild-types (WTs) with their SCVs
| Strain . | Clumping factor . | Coagulase . | DNAse . | Haemolysis . | Auxotrophy . |
|---|---|---|---|---|---|
| F1853 | |||||
| WT | + | + | + | + | |
| SCV | + | ± | < | − | − |
| F1854 | |||||
| WT | + | + | + | + | |
| SCV | + | + | < | − | − |
| F1855 | |||||
| WT | + | + | + | + | |
| SCV | + | − | << | − | − |
| Strain . | Clumping factor . | Coagulase . | DNAse . | Haemolysis . | Auxotrophy . |
|---|---|---|---|---|---|
| F1853 | |||||
| WT | + | + | + | + | |
| SCV | + | ± | < | − | − |
| F1854 | |||||
| WT | + | + | + | + | |
| SCV | + | + | < | − | − |
| F1855 | |||||
| WT | + | + | + | + | |
| SCV | + | − | << | − | − |
+, positive; −, negative; ±, weak reduction; <, reduced; <<, much reduced.
Comparison of the characteristics of wild-types (WTs) with their SCVs
| Strain . | Clumping factor . | Coagulase . | DNAse . | Haemolysis . | Auxotrophy . |
|---|---|---|---|---|---|
| F1853 | |||||
| WT | + | + | + | + | |
| SCV | + | ± | < | − | − |
| F1854 | |||||
| WT | + | + | + | + | |
| SCV | + | + | < | − | − |
| F1855 | |||||
| WT | + | + | + | + | |
| SCV | + | − | << | − | − |
| Strain . | Clumping factor . | Coagulase . | DNAse . | Haemolysis . | Auxotrophy . |
|---|---|---|---|---|---|
| F1853 | |||||
| WT | + | + | + | + | |
| SCV | + | ± | < | − | − |
| F1854 | |||||
| WT | + | + | + | + | |
| SCV | + | + | < | − | − |
| F1855 | |||||
| WT | + | + | + | + | |
| SCV | + | − | << | − | − |
+, positive; −, negative; ±, weak reduction; <, reduced; <<, much reduced.
Clumping factor: both wild-types and variants of all three isolates were clumping factor positive.
Coagulase: variants of F1854 showed no extension in time to produce a clot or reduction in clot quality. F1853 variant failed to produce a complete clot after 18 h, whereas F1855 variant produced no clot at all after 18 h.
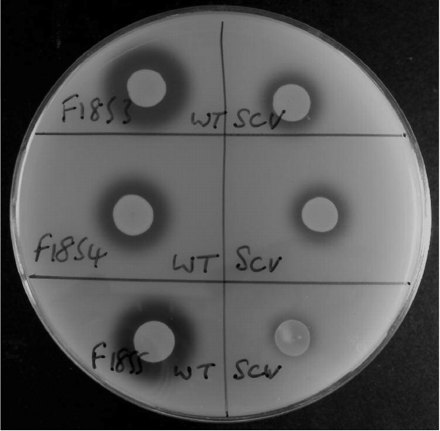
DNAse: variants showed considerably reduced DNAse production when compared with the wild-types; in the case of F1855, DNAse was almost completely absent (Figure 4).
Haemolysis: F1853, F1854 and F1485 wild-types were haemolytic for sheep blood. However, no haemolytic activity was seen from any of the three variants.
Auxotrophy: none of the variants showed auxotrophy for any of the substances tested.
Susceptibility to gentamicin: the MICs of gentamicin for the wild-types were 1.0 mg/L and for the SCV 0.125 mg/L. Re-incubation for a further 36 h did not make a difference to these results.
Triclosan susceptibility: the MIC of triclosan for each of the wild-types was 0.06 mg/L. The SCV of F1853, F1854 and F1855 showed an increase in MIC of 8-fold (0.5 mg/L), 17-fold (1.0 mg/L) and 67-fold (4.0 mg/L), respectively.
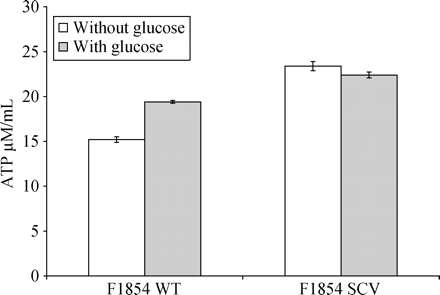
ATP: ATP content of the F1854 wild-type and variant, with and without glucose, is shown in Figure 5. In the absence of glucose, all three variants contained more ATP per cell than the wild-types. However, response to added glucose was uniformly positive (i.e. increase in ATP per cell) in all three wild-types, but negative (decrease in ATP per cell) in all three variants.
Reversion rate: none of the variants could be induced to revert to wild-type by repeated subculture on enriched medium or in liquid medium.
Suspensions of wild-type (WT) and SCV of MRSA strains F1853, F1854 and F1855 spotted onto DNAse agar. After development with 0.5 M hydrochloric acid, clear zones indicate DNAse production. Note the decrease in DNAse production in the SCV.
ATP content per bacterial cell of MRSA wild-type (F1854 WT) and its SCV form, after cultivation in TSB with and without added glucose.
Discussion
The method of impregnation of silicone with triclosan gives homogeneous distribution of the antimicrobial at a molecular level and sustained release for over 100 days.14 Although the method of introduction of triclosan into the polymers is different in commercial/domestic antimicrobial polymers, it still gives prolonged release and, therefore, prolonged exposure of bacteria to triclosan. In a healthcare setting, it is likely that MRSA would be exposed to such antimicrobial surfaces. Furthermore, although the use of triclosan-containing pharmaceuticals for handwashing or eradication of MRSA would afford only intermittent exposure, it is possible that their use over long periods (and possible contamination of dispensers, etc.) might give rise to resistant variants such as those described here. From the appearance and behaviour of the resistant variants, it is clear that they are SCVs, which are produced by many bacteria and were first reported in S. aureus from clinical sources.15,16 A concise definition of SCV is difficult to achieve, but one published requirement is that its colony size should be 10 times smaller than that of the parent strain.17,18 The strains described here gave rise to SCV having colony diameters approximately seven times smaller than the parents. However, there is considerable heterogeneity between strains of SCV, even from the same parent wild-type, and we and others have noticed a wide variation in colony size among SCVs. Certain S. aureus SCVs have been shown to be stable hemB mutants19 and these are particularly inducible by gentamicin, having been found colonizing gentamicin-loaded methacrylate beads and bone cement used in arthoplasty.20 SCVs seem to form a spectrum of response to stress, in this case due to exposure to triclosan. Proctor et al.21 have proposed that the common underlying factor in induction of SCV is a reduction in bacterial energy generation and/or transport, accounting for the down-regulation of such functions as cell wall synthesis and toxin production. This reduces the susceptibility of SCV to antibiotics and also enables them to evade phagocyte killing mechanisms.22,23 Each of these in turn leads to chronic persistent infections, which fail to respond to antimicrobial chemotherapy or which relapse after treatment.
SCV of S. aureus, therefore, could be expected to show a reduction in the production of haemolysin, coagulase and DNAse, to have reduced ATP content and to exhibit reduced susceptibility to the antimicrobial in whose presence they were generated. In addition, many but not all would show auxotrophy for haemin, menadione or other growth factors. Schaaf et al. reported that only 20% of spontaneous mutations to SCV showed auxotrophy.19 The variants reported here showed reduced haemolysin, coagulase and DNAse activity, but they did not show auxotrophy. They showed a significantly increased triclosan MIC, but paradoxically a reduced MIC for gentamicin. The latter is possibly due to the slow growth of the SCV, which might have produced a false result.24 The SCV isolated here showed more ATP per cell than the parents, as shown in Figure 5, but unlike the parent strains, none of the SCVs showed an increase in cellular ATP levels in response to glucose, suggesting impaired energy generation.
S. aureus SCVs have been isolated from chronic infections and they are clearly pathogenic25–28 and are able to evade antibiotics and to survive phagocytosis. In certain circumstances, many SCVs are able to revert to the fully toxigenic parent form, although we were unable to demonstrate this in our isolates. It is perhaps ironic that exposure to antimicrobial polymers containing an agent intended to reduce the risk of infection appears to generate a form of MRSA that is particularly well equipped to survive triclosan exposure, but is also likely to present considerable therapeutic problems if involved in infection. Although triclosan might yet prove useful in biomaterials for specific purposes under medical control, uncontrolled applications, especially in situations where long-term exposure might occur, could prove hazardous. It remains to be seen whether the ‘new’ antimicrobials directed towards the microbial fatty acid synthesis pathway,29 such as platensimycin,30 will give rise to SCV when used in MRSA infections.
Acknowledgements
We are grateful to the Wade Charitable Trust for general financial support and to The University of Nottingham for support for T. S.
Transparency declarations
None of the authors receives fees or remuneration in relation to the research reported here.