This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: S. Travis Altheide
, Eastern Kentucky University, travisaltheide{at}gmail.com
LEARNING OUTCOMES
1. Recognize different biochemical approaches to microorganism identification used in the clinical microbiology laboratory, including specific examples of tube-based, test kit, and automated methodologies.
2. Compare and contrast the advantages, disadvantages, and performance characteristics of each approach to identification, and determine the usefulness of each in addressing a specific clinical situation.
3. Identify the factors that drove the development of new biochemical technologies to address the evolving health care industry.
ABSTRACT
The late 1800s through the early 1900s saw the rapid development of growth media containing various substrates, that is, carbohydrates, to identify and differentiate microbes isolated from clinical specimens. However, during the 1950s, an evolution of diagnostic services occurred created by a growing population with access to health insurance and the subsequent requirement for quality health care. The utilization of miniaturized, multitest kits provided the first significant advancement of the biochemical, growth-based approach needed to handle the increased demand for clinical services. These testing kits provided reductions in labor and material costs associated with making, maintaining, inoculating, and reading tubed and plated media while also reducing the time required to identify microbial isolates. The trend to improve laboratory efficiency and quality continued with the incorporation of automation and computers throughout the 1970s. Automated systems greatly increased the testing capacity of laboratories by allowing the simultaneous determination of identification and antimicrobial susceptibilities from 1 inoculum and by further reducing the time to identification to hours instead of days. Within just the last 10–15 years, development and integration of a new growth-based approach for identification have occurred in the form of matrix-assisted laser desorption ionization time-of-flight mass spectrometry. The mass spectrometric approach provides the lowest cost per analysis and fastest time to identification after isolation of any current technique available in the microbiology laboratory. As health care costs and demand continue to increase and more hospitals look to consolidate laboratories, fully automated facilities incorporating mass spectrometry for identification, along with molecular methods, will become commonplace.
- API - analytical profile index
- AST - antimicrobial susceptibility testing
- MALDI-TOF - matrix-assisted laser desorption ionized time of flight
- MS - mass spectrometry
- PCR - polymerase chain reaction
- TSI - triple sugar iron
INTRODUCTION
The utility of the biochemical, or metabolic, test has been on display for nearly 150 years for the isolation and identification of microorganisms causing infectious disease due, in large part, to the very nature of microbial versatility. With the establishment of the germ theory of disease (1870), medical researchers were presented with a challenge to prove the link between microbe and disease. Instruction on how this could be accomplished came in the form of Koch’s postulates (1882), along with contributions from other researchers, with the specific requirement that a disease-causing agent must be isolated on culture media.1 Thus, what ensued was an age of great experimentation and discovery into the various growth properties of bacteria and other microbes, which was as reliant on knowledge of biochemistry as it was on standard microbiology techniques.2
DEVELOPMENT AND USE OF PLATE AND TUBE-BASED MEDIA FOR IDENTIFICATION
The need to cultivate and isolate infectious bacteria or fungal organisms led researchers to explore various nutrient-rich sources for organism growth. They began with the reasonable assumption that bodily fluids, and, by extension, basic organic components, such as protein and carbohydrates, would provide that source for growth. It was quickly observed that culture media containing blood, peptones, and even potatoes (among other ingredients) were quite effective for growing many different bacteria.2,3 In the late 1800s, the ability of certain bacteria to ferment particular carbohydrates, resulting in the formation of acid and gas, was noted to be a useful differential indicator for separating out members of the Enterbacteriaceae (referred to at the time as the colon-aerogenes family) and other gram negative bacilli from clinical and environmental specimens.4⇓-6 This led to the formation of the Durham tube, MacConkey agar, and eventually triple sugar-ferrous sulfate (now triple sugar iron [TSI]) media.4,7⇓-9 Other chemical reactions resulting in pH changes were observed and, by incorporating indicators in media, allowed detection of acidic and alkaline products, such as with citrate utilization10 and urease production.11
From 1900 through 1950 the enzymatic versatility of bacteria was harnessed in many single-test solid, semisolid, and liquid media, in which metabolic end products could be detected with the addition of a chemical reagent. Enzyme profiles through testing of nitrate reduction,12 indole formation,13 oxidase production,14 and amino acid utilization15,16 allowed laboratorians to differentiate among the ever-growing list of bacteria responsible for infectious diseases. Continued refinement of these diagnostic tests allowed for the development of rapid, or spot, testing for some compounds, resulting in quicker preliminary identification of enteric pathogens and common gram-positive isolates.14,17,18 Thus, by the middle of the 20th century, the biochemical, growth-based approach became a well-defined and effective methodology inside the diagnostic laboratory. Through the utilization of various growth substrates incorporated into solid and liquid media, a unique metabolic pattern of an unknown microorganism could be established, thereby providing a repeatable method for identification.
By 1965, the number of diagnostic tests utilized by the laboratory could range from 5 to over 20 depending on the type of bacterium isolated, the level of identification required (genus only or species), and the commonality of the organism.14,19 It became increasingly clear that the sheer volume of diagnostic tests, the increasing number of specimens submitted for identification, and the amount of data accumulated would require medical microbiology laboratorians to develop more efficient methods and technologies to handle the growing demand.14,20
IMPROVED EFFICIENCY THROUGH COMPUTATION AND MINIATURIZATION
As the population continued to increase from the turn of the century through the 1960s,21 so too did the demand for health care, hospital utilization, and laboratory services.22⇓-24 It was not only the increase in quantity of specimens but a demand for better quality of service, primarily due to the growth of public and private insurance, which required diagnostic laboratories to adapt methodologies and practices for improving efficiency.24,25 These methodologies mainly occurred through 2 avenues: computer-assisted identification and streamlining of tests through miniaturization.
To aid the bacteriologist in identification, flow charts, dichotomous keys, and diagnostic tables were usually consulted to guide one from preliminary through confirmatory testing. A step toward a more standardized process came in the form of cards containing a punched-out hole for each biochemical test.19 During the course of identification, the medical microbiologist would record their results by notching the holes for positive tests, then consult master cards obtained from reputable sources to match an identification. With the introduction of automatic data processing, the amount of physical material kept and time spent “looking up” an isolate were reduced, as were the errors associated with manually reading and matching results. Computing improved the accuracy of identification as well once databases were updated for newer tests and organisms. Computing improved accuracy through the use of probabilities analysis (Bayesian theorem), which allowed for determining the statistically most probable isolate from a collection of established results.20,26,27
At the same time, laboratories and researchers were collectively working to standardize and streamline testing procedures in order to more consistently differentiate microorganisms so time was not wasted on repeat testing or misinterpretation.20 Test miniaturization provided the next big improvement in laboratory efficiency in the form of multitest kits. Until the 1970s, the great bulk of required isolation and identification culture media were made in house at each diagnostic laboratory, requiring significant time, materials, and storage space19,28 and generating significant amounts of waste. Conventional plate- and tube-based testing, although versatile and effective for bacterial and yeast identification, could require approximately 8–20 tubes and take an average of 48–96 hours for complete interpretation after isolation, even for the more common aerobes and facultative anaerobes.14,19,24 Depending on the number of specimens and organisms submitted, the laboratory could have hundreds of tubes resting in incubators at any given time, creating a logistical headache along with the tedium of manually inoculating and reading such a high volume of tubes.24
Some of the first steps toward the multitest approach were conventional tube-based media containing 2 or more observable reactions (Table 1), such as Russell’s double sugar, Kligler’s iron, and TSI agars,8,29 and the use of paper discs or strips (PathoTec) impregnated with dried reagents.30,31 A 2-tubed (R-B media) identification system for members of the Enterobacteriaceae was designed to allow simultaneous testing of up to 8 biochemical reactions.32 A significant development in testing efficiency came from the work of Hartman, Buissiere, and Nardon, who established many of the physical and chemical requirements of a multitest micromethod.33,34 The outcome of this work produced the Analytab system (analytical profile index [API]), a plastic strip containing 20 small capsules or microtubes, with each capsule containing a dehydrated biochemical substrate for testing metabolic usage.35 From 1969 to 1979, a number of additional miniaturized multitest kits appeared in medical laboratories, including the AuxoTab, Enterotube, Micro-Media, and Micro-ID systems.36,37 Despite differences in design, the number of tests carried out, and incubation times, all of the multitest kits were based on adding a standardized inoculum to the system and observing for visible color changes in each testing compartment after incubation. These color changes were produced either spontaneously or after the addition of reagents.36
Summary of biochemical approaches and techniques discussed Abbreviations: API, analytical profile index; AST, antimicrobial susceptibility testing; ID, identification; MALDI, matrix-assisted laser desorption ionization; MS, mass spectrometry.
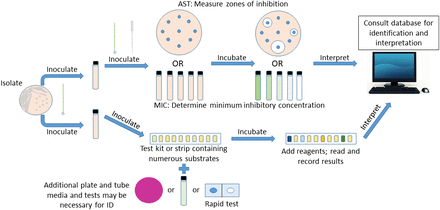
The miniaturized multitest approach brought many benefits and improvements to the diagnostic capabilities of the medical laboratory compared with conventional plate and tube-based identification. After the initial release of these systems, many underwent further refinement that improved both efficiency and accuracy. Ease of use and storage, it being readily disposable after use, it being accurate for identification, it being more economical in terms of data provided, and decreased time to identification were all advantages of this approach.35⇓⇓-38 The API system became the prototype kit adopted in many laboratories because of its versatility in usage for many groups of bacteria (Figure 1).
Scheme for the identification and antimicrobial susceptibility testing of a clinical isolate using a miniaturized test kit approach.
Utilizing the API 20E system instead of conventional tube-based testing for members of the Enterobacteriaceae reduced by more than half the total cost per identification of an isolate, and this was accomplished through a reduction in both labor and media.39 For common gram-positive and gram-negative bacteria, the API gave ≥90% congruence with conventional biochemical testing methods, making it a reliable alternative.40⇓-42 Additionally, other kits were developed for the typically hard-to-grow and test species of Streptococcus, Neisseria, and Haemophilus. Subsequent development of rapid test kits allowed reproducible results obtained more quickly for testing of common anaerobes as well as other fastidious organisms, with a time to identification in the range of 4–48 hours.28,43,44
MAXIMIZING EFFICIENCY WITH AUTOMATION
As the number of individuals seeking quality health care continued to rapidly increase, so too did the cost of providing required health care, driving the demand for quicker, more efficient, and more accurate microbiology diagnostic services.25,45 Although utilizing multitest kits provided many advantages as previously outlined, there were still opportunities to improve upon the formula. Miniaturized kits required manual inoculation, addition of external reagents, and interpretation of results, which were prone to inconsistences and errors, requiring further time to complete. The API 20E system required at least 3 minutes to inoculate and 10 minutes to interpret after incubation per test36,38; kits providing more tests could take even longer. When only a few samples are involved, this time requirement is fairly insignificant. However, inoculating and reading 50–100 samples a day would require substantial labor and time in comparison.
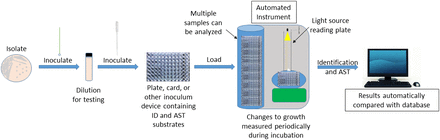
Automatic data processors had demonstrated their efficiency and effectiveness for years and were now widely available to medical laboratories. It now became possible to explore the utility of automation in bulk microbial identification. The AutoMicrobic System (Vitek), born out of a National Aeronautics and Space Administration initiative to identify microorganisms in a spacecraft environment, was subsequently modified in 1973 to identify clinical isolates directly from urine specimens.46 Although successful identification rates were high, it was not cost effective to run every specimen directly through the system without isolation and screening procedures to eliminate unnecessary reagent waste and technician time.47 This system was further modified to run identification tests from a standardized inoculum containing 1 organism. From the late 1970s through the early 2000s, additional automated and semi-automated systems based on metabolic and enzymatic analyses were developed and further refined, including, but not limited to, MicroScan, Phoenix, Sensititre, and Vitek systems (Table 1), each with their own custom substrate cartridge, kit, panel, or plate for testing.48⇓-50
Fully automated instruments are responsible for the majority of specimen identification in hospitals today. They can vary slightly or considerably from one another in their degree of automation, spectrum of organisms identified, method of inoculation, sample capacity, and turnaround time.51⇓-53 Automated instruments use turbidometric, colorimetric, and/or fluorimetric principles for quicker and, usually, more statistically confident identification of isolates compared with any manual, kit-based method.50,51,54 More so, automated methods, regardless of the particular instrument used, have increased the total number (>200) of clinical isolates that can be inoculated, incubated, and identified within the span of 24 hours. Thus, laboratory productivity is increased without sacrificing quality of results.52,54,55 An equally, if not more, significant advancement with automated biochemical systems over their manual counterparts is the ability to simultaneously determine an unknown isolate’s identification and antimicrobial susceptibility patterns in the same device under the same condition (Figure 2).
Scheme for the identification and antimicrobial susceptibility testing of a clinical isolate using a standard automated biochemical approach.
With the widespread use of antibiotics and the concurrent observation of antimicrobial resistance, the need to conduct antimicrobial susceptibility testing (AST) became a necessity. AST was initially developed and standardized through the Kirby-Bauer,56,57 or disk diffusion, method. Standardized macrodilution and microdilution methods for minimum inhibitory concentration determination occurred in the late 1970s.58 Before automated instrumentation became widely available, all AST was accomplished manually and separately from the identification of an isolate (Figure 1), requiring additional materials and added time both in set-up and in obtaining results: a minimum of 18–24 hours compared with as little as 3.5–18 hours.59,60 The decrease in turnaround time for both identification and antimicrobial susceptibility determination ultimately led to a shorter length of stay for patients (~1 day shorter) and decreased mortality rates, resulting in substantial cost savings through a reduction in additional laboratory tests and performance of fewer invasive procedures.60,61 Today’s automated instruments come with extensive databases and analytics software that allow further scrutiny of AST results for detection of resistance and atypical patterns while providing the ability to communicate information faster with pharmacy personnel to deliver real-time adjustments to patient care.62,63
Currently, automated and semi-automated biochemical testing are the most widely utilized approach to microbial identification and antimicrobial susceptibility determination for the majority of bacterial and yeast isolates.64 Additionally, automated modular machines, including Kiestra and WASP, have been developed to handle the processing, inoculation, and incubation of bulk, routine specimens, such as urine and swabs, as well as provide growth imaging and isolate selection.65 Nevertheless, automated technologies have not simply replaced all prior biochemical methodologies, regardless of laboratory capacity.
Prepackaged, multitest kits and tube-based media are still routinely used for fastidious, hard-to-grow, and rarely encountered organisms, including Viridians streptococci, species of Neisseria and Haemophilus, some nonfermenting gram-negative rods, Vibrio species, mycobacteria, and anaerobes, which may not be identified accurately or, in many cases, are unable to be identified, with automated methods.44,52,55,66⇓-68 Other, nonmetabolic-based methods, such as serology and molecular, have provided additional approaches to microbial identification without the need for isolation and growth of problematic organisms. Moreover, determining the antimicrobial susceptibility patterns of these same microorganisms, when necessary, requires nonautomated methods as well, such as disk diffusion and Etest (bioMerieux), for accurate results.63
A NEW PARADIGM IN CULTURE-BASED TESTING
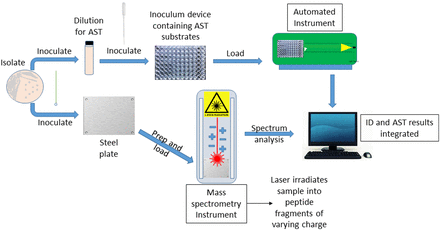
With growth-based biochemical methods handling the bulk identification of common bacteria and yeast, can we expect any further advancement in application and instrumentation within this approach? The development of chromogenic media, refinement of testing panels, improved optics, and more robust software analytics have provided some increase in efficiency and accuracy in recent years.68,69 Although it is hard to anticipate future innovation, it is unlikely that we will see such significant improvement of current biochemical diagnostic techniques as have occurred with the transition from tube-based testing to miniaturized test kits and, finally, to full-scale automation. The limiting factor here is the dependency on microbial growth and substrate utilization for identification. Use of mass spectrometry (MS) for the identification of pathogenic bacteria (Table 1), first described in 1975, represents a unique phenotypic approach that has recently become a very practical method for speciation of a wide array of microorganisms in the clinical setting.70⇓⇓-73
Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS is not a traditional biochemical technique; organisms are not grown with various substrates to determine metabolic usage, and specific enzyme production is not detected. Best described as an analytical chemistry identification method, MALDI-TOF determines an organism’s protein “fingerprint” by bombarding a single colony with a high-energy laser to produce individual peptide fragments that vary in size, yielding a unique chemical spectrum.74 It is not the first non–utilization-based method used for identification; gas and liquid chromatography techniques were developed in the 1980s and 1990s to identify bacteria and yeast based on their cellular composition.51,64,75 However, MALDI-TOF is a true departure from any other phenotypic approach in its functional, efficient, and accurate application to nearly all groups of microorganisms.76 This approach has been shown to consistently perform, at the very least, as well as automated biochemical methods, though usually better.77 Indeed, mass spectrometry is the most likely technique to completely replace current automated, growth-based systems for routine identification of isolates, as has already occurred within many diagnostics laboratories (Figure 3). This includes for the identification of rarely encountered, fastidious, and slow-growing organisms, such as species of Campylobacter and Helicobacter,78 Legionella,79 Mycobacterium,80,81 anaerobes,82 nonfermenting bacteria,83,84 yeasts85,86 and filamentous fungi.87,88
Scheme for the identification and antimicrobial susceptibility testing of a clinical isolate using the MALDI-TOF approach.
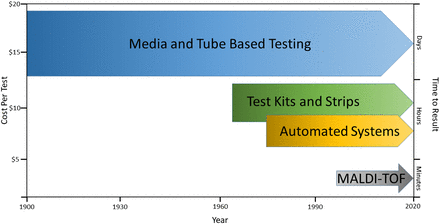
The capability to accurately and precisely identify bacteria and fungi at an extremely low cost-per-sample analysis is yet another advantage of the mass spectrometric approach (Figure 4). Long- and short-term studies have shown significant reductions in costs when comparing conventional and automated approaches with MALDI-TOF MS.73,76,85 One 11-year study demonstrated a 5-fold and a nearly 100-fold reduction in the average cost per test compared with current phenotypic and gene sequencing methods, respectively.83 Other studies have supported these findings, revealing an approximate cost of $0.50–$2.00 for each identification, depending on the specific instrument used and the type of isolate.73,89,90 The initial cost of an MALDI-TOF MS instrument can be a limitation for smaller laboratories with a price range of $180 000– $200 00089; however, the savings from lower operating and labor costs and a reduction in repeat testing, subculture media, and waste can offset the initial cost within 1 year depending on the number of samples routinely analyzed.77,91
Qualitative comparison of biochemical and growth-based approaches to identification. Cost per test includes materials and labor as estimated by studies cited and discussed in the text.
Without the need for microbial growth after isolation, time to result is greatly reduced compared with conventional methods as well (Figure 4), with the majority of identification results (87%–93%) obtained 12–24 hours after receipt of the initial specimen.91,92 Once a sample is placed on the instrument, identification typically occurs within 5–10 minutes depending on the organism type.74,89,92 The cost- and time-saving advantages of using MALDI-TOF MS are most pronounced for the workup and identification of slow-growing bacteria, specifically anaerobes and mycobacteria, in which a result may be obtained within minutes after isolation instead of days or weeks.
Limitations of MALDI-TOF MS in identifying some organisms are largely due to a lack of sufficient reference spectra in databases.64,83 Currently, databases are proprietary, unlike databases of genetic sequence, so they require manufacturer updates to enhance detection limits.64 Laboratories may build their own in-house databases to further improve the instruments capabilities, though the quality of spectra obtained must be ensured through molecular means to prevent spreading of erroneous data.77,89 Some organisms, such as E. coli and Shigella species, may not be reliably identified owing to nearly identical genetic profiles and, thus, nearly identical protein fingerprints. Human error, inadequate training, culture conditions, and media composition are also sources of potential result variability with MALDI-TOF, as the current iterations of this technique require specific preanalytical techniques depending on the type of microorganism being tested. Additionally, laboratories currently using MALDI-TOF MS as the primary method of identification must still rely on automated biochemical instruments and manual methods for determining antimicrobial susceptibilities.
FUTURE APPLICATIONS AND PROSPECTS
The requirement to first isolate an infectious agent from a clinical sample is always required with any current biochemical and growth-based approach, including mass spectrometry in its current state. As such, there is still some inherent risk when handling select agents and some organisms that cannot be isolated or are very difficult to isolate still requires other means of testing.89 Serological and molecular techniques provide the means to test for organisms directly from specimens, thereby providing a potentially quicker identification while avoiding the inherent difficulties and risks of isolating and growing especially dangerous or fastidious microbes.
Nevertheless, MALDI-TOF MS has been shown to reliably identify organisms directly from some clinical specimens, including blood and urine, whereas other specimen types are currently being investigated.89,93 Likewise, there is potential for MALDI-TOF MS to differentiate individual strains of microorganisms as well as detect antimicrobial resistance and resistant mechanisms, including β-lactamase and carbapenemase production, and detection of methicillin-resistant Staphylococcus aureus protein-specific spectra.94,95 It is also possible to apply the mass spectrometric approach to other biomolecules, such as base nucleotides, to produce new methodologies like polymerase-chain-reaction (PCR) electronspray ionization MS.96 This hybrid technique combines the speed and utility of MALDI-TOF MS with the sensitivity and specificity of PCR amplification. Future advancements and applications will likely focus on incorporating these existing technologies into a fully integrated and automated system, from processing, to incubation, to isolation, and to identification so as to reach maximum operational efficiency by further reducing costs and turnaround time. Further work is necessary to improve the efficiency and practicality of these particular MALDI-TOF MS applications in order for them to be adopted for routine clinical usage.
CONCLUSIONS
The biochemical and growth-based approaches in the diagnostic laboratory were initially born out of the discovery of microorganisms and the need to confirm their causative link to infectious disease. As the need and requirement for quality health care became commonplace, laboratories improved diagnostic capabilities and increased testing capacity without sacrificing quality of results by first utilizing miniaturized, multitest kits followed by computer automation for the routine identification of clinical isolates. From their initial inception in the late 1800s to within just a few years ago, growth-based, biochemical identification methods have been the de facto method of choice for hospital-based microbiology laboratories. Now we have entered a new era of diagnostic testing wherein the routine approach is based not on what an organism’s proteins do but by the proteins themselves.
A laboratory’s capacity to adopt new methodologies and technologies is dependent on many factors though, and newer approaches, such as MALDI-TOF, will not, and cannot, be utilized by every microbiology department, as it may not be cost effective or practical for smaller-capacity hospitals. However, with the continual increase in cost of and demand for health care services coupled with the level of accuracy and efficiency offered by MALDI-TOF and other newer techniques, more and more laboratories will make the change. As diagnostic laboratories continue to consolidate into fewer but larger testing facilities embracing full-scale automation, traditional approaches and methods will continue to be replaced.97 Although medical laboratory technicians and scientists have always needed to acquire new skills and knowledge to use new methods and tools as they become available, the underlying principle behind these techniques has remained relatively unchanged until very recently. It is becoming more likely that the instilled art and practice of memorizing and reading biochemical growth patterns will no longer be required of the diagnostic microbiologist.
ACKNOWLEDGMENTS
I would like to thank my fellow focus series authors for their input concerning this manuscript. I would also like to thank an anonymous reviewer for their comments and suggestions, which made this a better paper.
- Received July 16, 2019.
- Accepted January 7, 2020.
American Society for Clinical Laboratory Science
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵