This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Larry J. Smith, PhD, SH(ASCP), HCLD/CC(ABB)⇑
- Address for Correspondence: Larry J Smith, PhD, SH(ASCP), HCLD/CC(ABB) Abbott Diagnostics Division – Hematology Unit, 4551 Great America Pkwy, Santa Clara, CA 95054
Abstract
OBJECTIVE: This review describes consensus guidelines and laboratory methods for diagnosing a lupus anticoagulant (LAC).
BACKGROUND: A prolonged APTT due to the presence of a LAC is a frequent finding in the clinical laboratory in patients referred for preadmission testing. Accurate detection of the LAC can often be very challenging for the laboratory. While no single assay is specific for diagnosing a LAC, there is a combination of assays that can be used to improve laboratory diagnosis.
METHOD: This review describes several assays used to detect a LAC; reviews consensus guidelines that influence the choice of assays by laboratories; and associated preanalytical variables that may lead to a missed diagnosis.
CONCLUSION: A thorough understanding of the principles involved in LAC testing and preanalytical variables may lead to a more accurate diagnosis of the LAC.
ABBREVIATIONS: aB2GPI - anti-B2 glycoprotein I, aCL - anti-cardiolipin, APLAs - antiphospholipid antibodies, BCSH - British Committee for Standards in Haematology, CLSI - Clinical and Laboratory Standards Institute, DOAC - direct oral anticoagulant, dRVVT - dilute Russell's viper venom time, ISTH-SSC - International Society for Thrombosis and Haemostasis – Scientific Standardization Committee, LAC - lupus anticoagulant, VKA - vitamin K antagonist, SCT - silica clotting time.
- INDEX TERMS
- Antiphospholipid antibody
- anticardiolipin antibody
- beta-2 glycoprotein I antibody
- lupus anticoagulant
- dilute Russell's viper venom time
- silica clotting time
- STACLOT-LA
INTRODUCTION
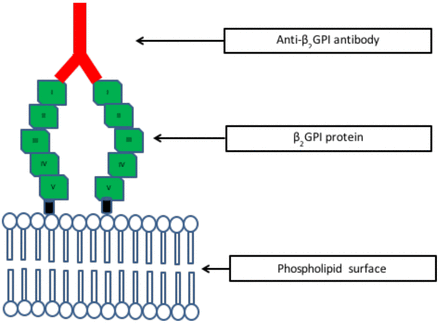
The ability for a laboratory to accurately detect the presence of a lupus anticoagulant (LAC) is critical when evaluating patients with thrombotic disorders and/or recurrent spontaneous abortions. Often physicians will order antiphospholipid antibody testing alone or in conjunction with other tests as part of a thrombophilia panel. Antiphospholipid antibodies (APLAs) are a group of heterogeneous circulating autoantibodies that include LAC, anticardiolipin antibodies (aCLs), and anti-β2-glycoprotein antibodies I (aB2GPI).1 These autoantibodies are directed against anionic phospholipids or proteins associated with phospholipids and result in prolongation of the activated partial thromboplastin time (APTT) and some other assays in the laboratory. The best characterized of these proteins are β2-glycoprotein I (B2GPI) and prothrombin (Figure 1).2 The presence of these antibodies is significant, because they are associated with a prothrombotic state rather than bleeding in most cases. When the antibodies are present, they are often associated with the antiphospholipid syndrome (APS), which is an autoimmune disorder. Individuals with APS present with arterial and/or venous thrombosis and recurrent spontaneous miscarriages. These antibodies are found in about 5% of normal individuals, and approximately 30-40% of patients with systemic lupus erythematosus (SLE). Of the patients with SLE, only about 10% actually have APS.3,4 Fifteen to 20% of patients with APS develop deep vein thrombosis (DVT) and/or pulmonary embolism (PE). In addition, 25% of women with recurrent miscarriages and about 1/3 of stroke patients under the age of 50 are diagnosed with APS.5 The diagnostic criteria for APS includes one clinical event and one laboratory criterion. The clinical event includes an arterial or venous thrombotic occlusion or an obstetrical complication. The laboratory criteria includes a positive cardiolipin antibody (IgG and/or IgM), a positive B2GPI antibody (IgG and/or IgM), or a positive LAC assay. The laboratory event must be positive on at least two occasions about 12 weeks apart.6 Testing for APLAs consists of solid phase assays on an immobilized surface (aCLs and aB2GPI) and functional assays for the LAC. This review will focus on LAC testing only.
The LAC was initially described in 1952 by Conley and Hartmann in two patients with systemic lupus erythematosus (SLE) who presented with a bleeding diathesis.7 They called it “lupus” since it was described in both of these patients with a diagnosis of SLE and “anticoagulant” since both of these patients presented with bleeding. The LAC is somewhat of a paradox, because the majority of patients who present with a LAC experience thrombosis rather than bleeding. Thus, the term “lupus anticoagulant” is a double misnomer. Not all patients with SLE have a LAC, and even though the LAC prolongs the clotting time, it generally does not cause bleeding in vivo.
Pathophysiology of the LAC
LACs are a group heterogeneous autoantibodies (IgG and/or IgM) found in the circulation that bind to negatively charged phospholipid-binding proteins such as B2GPI and prothrombin (Figure 1).8 Phospholipid surfaces are required for coagulation to occur. When coagulation complexes (extrinsic, intrinsic tenase complex, and prothrombinase complex) form on these surfaces, thrombin is generated and leads to fibrin formation. When LAC's are present in plasma, they prevent these coagulation complexes from attaching to the phospholipid surface in in vitro coagulation assays where a limited amount of phospholipid is present in the reagent. As a result of the limited phospholipid concentration in the reagent, phospholipid-dependent clot based assays such as the APTT are prolonged. APTT reagents differ in their phospholipid concentration, which contributes to the reagent's sensitivity to a LAC. Therefore, depending on the phospholipid concentration, some APTT reagents will be more sensitive to the presence of a LAC resulting in a prolonged APTT (decreased phospholipid concentration), while others may not be as sensitive resulting in a normal APTT (increased phospholipid concentration). This prolongation does not occur in vivo since there is an unlimited source of phospholipid on platelets and erythrocytes in the circulation and on the endothelium. Therefore the LAC is a paradox that leads to prolongation of the clotting time in vitro and thrombosis in vivo.
β2GPI and antibody. β2GPI and antibody to β2GPI binding to a phospholipid surface. B2GPI consists of 5 homologous domains. Domain V binds to the anionic phospholipid surface. The anti-β2GPI antibody binds to domain I.
There are a number of mechanisms that have been proposed to explain how LACs cause thrombosis in vivo. Some of these include inhibition of activated protein C (APC); disruption of thrombin regulation; altered fibrinolysis; and endothelial cell activation and disruption.9 APC down regulates thrombin generation by inhibiting FVa and FVIIIa. It has been suggested that the anti-B2GPI antibody complex (aB2GPI) may compete with APC for phospholipid binding or disrupt the interaction between APC and the phospholipid binding site.10 In vitro studies have demonstrated that B2GPI (domain V) may prevent thrombin inactivation via heparin cofactor II (HCII) by binding to exosite II on thrombin. This prevents the heparin:HCII complex from binding to thrombin. As a result, thrombin is not inhibited by the heparin:HCII complex. A murine aB2GPI monoclonal antibody directed against B2GPI (domain I) has been shown to significantly enhance this inhibitory effect.11 These monoclonal antibodies have been shown to also interfere with plasmin activity and annexin A2.12 Annexin 2 brings tissue plasminogen activator and plasminogen together on tissue leading to plasmin generation. Interfering with plasmin generation leads to decreased clot dissolution.9 Endothelial activation and disruption has been shown to stimulate inflammatory and procoagulant responses that lead to thrombosis and the release of procoagulant microparticles into the circulation, respectively.9,13
Laboratory tests
The clinical laboratory plays an important role in the diagnosis of a LAC. However, there are a number of differences in laboratory practices in terms of the choice of LAC assays, practices and outcomes.14 In order to improve diagnostic accuracy of LAC testing, expert panels have proposed guidelines for laboratories to follow that cover preanalytical, analytical, and post analytical phases of LAC testing. The guidelines have been proposed by: 1) the International Society of Thrombosis and Haemostasis Scientific Standardization Committee (ISTH SSC), which published revised guidelines in 2009; 2) the British Committee for Standards in Haematology (BCSH), which published guidelines in 2012; and 3) the Clinical and Laboratory Standards Institute (CLSI), which published guidelines in 2014.15-17
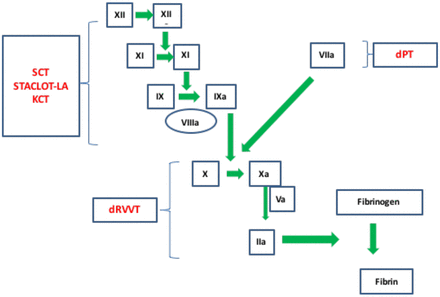
There are several different LAC assays performed in clinical laboratories for the identification of a LAC. These include the dilute Russell Viper Venom Test (dRVVT), Silica Clotting Time (SCT), Hexagonal Phase Phospholipid Neutralization (STACLOT-LA), Kaolin Clotting time (KCT), dilute Prothrombin Time (dPT), Platelet Neutralization Procedure, Tissue Thromboplastin Inhibition, and two less commonly used assays, the Taipan Venom Time, and Textarin:Ecarin Ratio, etc.18 Some of the more commonly performed LAC assays and where they interact in the coagulation cascade can be seen in Figure 2. Since no single LAC assay will detect all LACs due to the heterogeneity of the LAC antibody, all three expert panels recommend that laboratories perform at least two different assays based on different test principles. This review will focus on three of the more common assays: dRVVT, SCT, and Hexagonal Phase Phospholipid Neutralization (STACLOT-LA).
Suspicion for the presence of a LAC generally starts with an unexplained elevated APTT. This is followed by a 1:1 mixing study of patient plasma with normal plasma. If the clotting of the 1:1 mix corrects, it suggests a factor deficiency. If it does not correct, it suggests a circulating inhibitor. If there is no correction in the 1:1 mixing study and no reported history of bleeding in the patient, physicians generally request a work-up for a LAC. This work-up can involve a single assay (such as the dRVVT, SCT, or STACLOT-LA) or a panel of assays. The International Society of Thrombosis and Haemostasis Scientific Standardization Committee (ISTH/SSC) published revised guidelines in 2009 recommending that laboratories perform: 1) at least 2 screening tests that demonstrate prolongation of a phospholipid-dependent clotting time based on different test principles, (2) a mixing study to confirm the presence of an inhibitor and exclude a factor deficiency, (3) a confirmatory test that demonstrates phospholipid-dependence of the inhibitor, and (4) rule out other causes of prolongation that may be due to a specific factor inhibitor or the presence of drugs.15
Interaction of specific LAC assays within the coagulation cascade. The heterogeneous nature of the LAC requires multiple assays to improve diagnostic sensitivity. (dPT, dilute Prothrombin Time; dRVVT, dilute Russell Viper Venom Time, KCT, Kaolin Clotting Time; SCT, Silica Clotting Time).
Dilute Russell Viper Venom Time
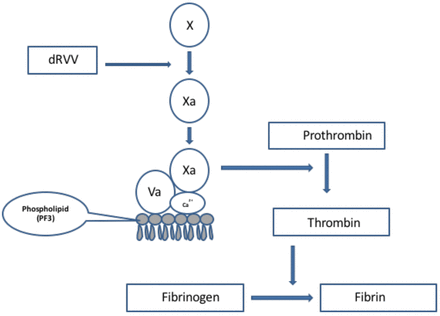
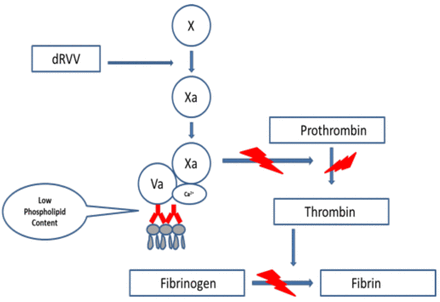
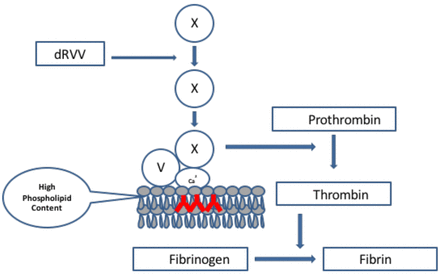
The dRVVT is recognized by all three expert groups as one of the initial assays to be included when screening for a LAC. It has been shown by a number of investigators to be sensitive to B2GPI-dependent antibodies and to correlate very strongly with thrombosis.8 This assay is based on the activation of FX at the beginning of the common pathway by an enzyme derived from Russell viper (Daboia russelii) venom. In the presence of phospholipids, calcium ions and FVa, FXa converts prothrombin to thrombin. Thrombin then converts fibrinogen to fibrin. The dRVVT assay is not affected by deficiencies located in the extrinsic or intrinsic pathways of the coagulation cascade (Figure 3). The dRVVT usually consists of two components. The first component is a screening reagent that contains a decreased amount of phospholipids in the reagent making it very sensitive to the presence of a LAC (Figure 4). When patient plasma containing a LAC is added to the screening reagent, the clotting time is prolonged, because the antibodies present in the plasma interfere with the prothrombinase complex's ability to bind to the phospholipid surface (Figure 4). The second component is a confirmatory reagent, which has an increased amount of phospholipid added to the reagent. The additional phospholipids neutralize the antibody by providing an increased surface area for binding of the prothrombinase complex. This leads to a shortening of the clotting time compared to the screening assay (Figure 5). When the screen and confirm are used together, they can be considered an “integrated” assay.19 The dRVVT has been shown in several studies to be specific for detecting LAC in patients with a high risk for developing thrombosis and more sensitive than the KCT.8,20,21 The dRVVT may give false positive results in patients on vitamin K antagonists (VKA). Antibodies to FV and/or FV deficiency have also been reported to interfere with the assay.18,22 It is recommended that laboratories calculate a dRVVT screen ratio (patient dRVVT screen result/mean of dRVVT screen normal range (NR) and a dRVVT confirm ratio (patient dRVVT confirm/mean of dRVVT confirm NR) and report the integrated result as a normalized ratio (dRVVT screen ratio/dRVVT confirm ratio).19,22 In general, a ratio >1.2 is suggestive for the presence of LAC.22
Silica Clotting Time
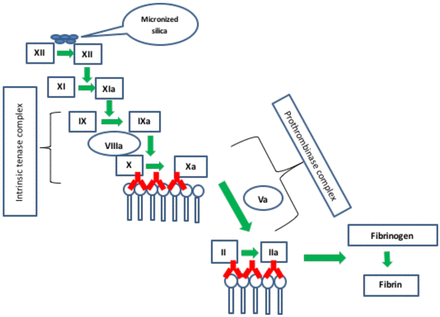
The SCT (WERFEN, Barcelona, Spain) is another integrated test system that consists of two components similar to the dRVVT. A colloidal silica suspension is used in the reagent to activate FXII in the intrinsic pathway (Figure 6). Patient plasma to be investigated for LAC is added to the reagent, which contains a low concentration of phospholipid. The low concentration of phospholipids increases the sensitivity of the reagent to the presence of a LAC resulting in a prolonged clotting time. In the confirmatory phase of the assay, patient plasma is added to a second reagent containing an increased amount of phospholipid. The additional phospholipid neutralizes the antibody by providing increased surface area for the intrinsic tenase and prothrombinase complexes, which shortens the clotting time (Figures 4 and 5). This assay was shown in some studies to be the most sensitive assay for identifying LA in patients who meet the clinical criteria for APS.23 The assay may be insensitive to VKA's and specific antibodies to FVIII may interfere with the interpretation of results.18 It is recommended that laboratories calculate both an SCT screen ratio (patient screen result/mean of screen NR) and a SCT confirm ratio (patient confirm result/mean of confirm NR) and report the integrated result as a normalized ratio (screen ration/confirm ratio)24. In general, a ratio >1.16 is suggestive for the presence of a LAC.24
Mechanism of action of dRVV on the coagulation cascade. The enzyme present in Russell viper venom converts FX to FXa. FXa in the presence of FVa, calcium ions and phospholipids converts prothrombin to thrombin. Thrombin converts fibrinogen to fibrin.
Hexagonal Phase Phospholipid Neutralization
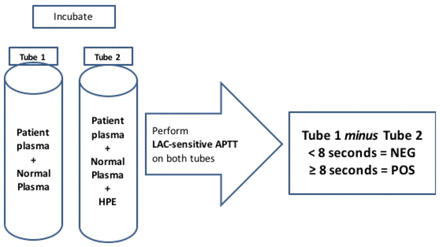
The Hexagonal Phase Neutralization, or STACLOT®LA (STAGO, Asnières sur Seine, France) is an integrated assay where patient plasma is mixed with normal control plasma and incubated in the presence and absence of a hexagonal phase (II) phosphatidylethanolamine (HPE) phospholipid. An APTT is then performed on both samples simultaneously using a LAC sensitive reagent (low phospholipid concentration) (Figure 7). If a LAC is present, it will be neutralized by the HPE resulting in a shorter clotting time compared to the sample incubated in the absence of HPE. The purpose of the normal plasma used in the patient mixture is to correct for a factor deficiency if one were present. If there is no shortening of the clotting time in the tube containing HPE, then a specific factor inhibitor rather than a LAC should be suspected. Results are reported as the difference in clotting time between the tube without HPE minus the tube containing HPE. This assay is not affected by VKA's.25 Laboratories should determine their own cut-off value, but in general, a difference ≥ 8 is suggestive for the presence of a LAC.26
DRVVT screening assay. The screening assay requires a lupus sensitive reagent that contains a decreased amount of phospholipid. If a LAC is present, it will interfere with the prothrombinase complex binding to the phospholipid surface of platelets.
DRVVT confirm assay. The confirm assay requires a reagent that contains an increased concentration of phospholipid. If a LAC is present, it will neutralize the antibody facilitating binding of the prothrombinase complex onto the platelet phospholipid surface.
Mixing tests
The APTT mixing test is frequently requested to screen for the presence of an inhibitor or a factor deficiency when there is an unexplained elevated APTT. Patient plasma (PP) is mixed with pooled normal plasma (PNP) and the sample is tested immediately and after incubation for 60-120 minutes at 37°C in a water bath. There are various mixing ratios of PP to PNP that can be used, however most commonly a 1:1 ratio is used. When the mixing test fails to correct in both the immediate mix and the incubated mix, it suggests the presence of a circulating inhibitor. In the case of a LAC, the inhibitor is identified by using a specific LAC assay. Caution must be exercised when interpreting mixing tests, because a weak inhibitor may be masked when PNP is added to PP. “Correction” in mixing tests varies among laboratories. Some laboratories may choose to express correction relative to the normal APTT reference interval (RI) (within 2 to 3 standard deviations of the RI) or a predetermined number of seconds above the upper limit of the RI. Laboratories may also choose to use a formula to calculate a “percent of correction” of the patient's clotting time plasma based on an index of circulating anticoagulant (ICA), also known as the Rosner Index.17, 27 This compares the degree of correction in the mixing test to the patient's baseline clotting time.17 The CLSI guidelines (H60A) do recommend reporting mixing tests as a normalized ratio rather than as clotting times in seconds.17
Silica Clotting Time assay (SCT). Micronized silica is used to activate the intrinsic pathway. LAC antibodies (in red) interfere with binding of the intrinsic tenase and prothrombinase complexes on the platelet surface.
The mixing test can be performed as a stand-alone mixing test, as described above, or integrated into the dRVVT or SCT. It is already an integral component of the STACLOT-LA. The integrated mixing test is performed on the immediate mix only and is never incubated. All three expert panels include the mixing test in their guidelines. Some recent guidelines suggest that the integrated test does not need to be incorporated into the mixing test when the test result is reported as a normalized ratio against PNP.25 However, some investigators believe that the integrated mixing test helps to avoid false-positives in patients with a prolonged dRVVT confirm assay, and suggest that when the results are from patients receiving VKA's, that they be interpreted with caution.26 Although rare, the lupus cofactor effect, a phenomenon where by the 1:1 mix is more prolonged than the patient sample, has been reported in cases where ellagic acid is used as an activator.27 Loeliger et al associated this prolongation with a decreased to absent level of prothrombin in the index plasma resulting in an immune mediated response that occurred during mixing with normal plasma.28 Some investigators believe that this cofactor effect can only be demonstrated with a mixing test. Thus, there are still differences of opinion concerning the use of mixing tests.
Hexagonal Phospholipid Neutralization assay (STACLOT-LA). Patient plasma plus normal plasma are incubated in the absence of HPE tube 1) and in the presence HPE (tube 2). An APTT is performed using a LAC sensitive reagent. The clotting time of tube 1 is compared to tube 2. If a LAC is present, tube 1 minus tube 2 should be ≥ to the established cut-off.
Preanalytical variables
There are a number of variables (preanalytical, analytical, and post-analytical) that interfere with LAC testing. Therefore, proper sample collection and preparation is important. Platelets (fresh or freeze/thawed) contain anionic phospholipids that neutralize the effect of the LAC. When preparing samples for processing, blood should be centrifuged at 2000 g for 15 minutes at room temperature, plasma transferred to a new tube, and recentrifuged at > 2500 g for an additional 10 minutes to ensure platelet free plasma (<10 x 109/L).15 If plasma is to be tested at a later date, samples can be stored at -70°C for up to 6 months in and then thawed rapidly at 37°C ± 1 degree for 5 minutes in a water-bath.
APTT reagents differ in their sensitivities to a LAC, because they contain different concentrations of phospholipids and different types of contact factor activators.14 Phospholipids may be prepared from animal or plant sources or may be a synthetic preparation. The phospholipid structure may be bilayer (lamellar) or hexagonal. The activators used in the reagents are negatively charged substances such as celite, ellagic acid, kaolin, micronized silica, etc. Reagents also vary in their sensitivities to the presence of heparin, vitamin K antagonists (VKAs) and direct oral anticoagulant (DOAC) drugs. Ideally, patient samples should be tested in the absence of all of these interfering substances. Most experts agree that LAC testing should not be performed in patients taking VKAs; there are studies to suggest that the normalized ratio from some tests such as the dRVVT may allow for diagnostic testing to be performed.30 In addition, when a mixing test is performed as a part of an integrated assay, it should theoretically minimize the effect of VKAs. In the case of heparin, most LAC reagents contain a heparin neutralizing agent to lessen the interference of heparin over a therapeutic dose range. The DOAC's, both anti-IIa (Dabigatran) and anti-Xa (Rivaroxaban, Apixaban, Edoxaban) have all been shown to possibly lead to a false-positive classification of a lupus anticoagulant.31 To avoid the chance of interference by anticoagulant drugs, it is preferable to test patient samples in the absence of all anticoagulant drugs. All of these variations in reagents lead to inconsistencies when reporting LAC results. In addition to different reagent composition, differences in clot detection systems on coagulation analyzers may further complicate the issue. As a result of all of these variations, there is a need for standardization in LAC testing and reporting among laboratories.14
Specific inhibitors to the coagulation factors have also been associated with false positive LAC results. These can be identified by mixing studies and by performing specific factor assays when performing LAC testing. A Bethesda titer can be used to quantitate the antibody titer when indicated.
Guidelines and Recommendations
To address some of the issues in standardization for LAC testing, the three expert panels previously mentioned have created guidelines and recommendations based on “best” practice to improve the diagnostic accuracy of LAC testing and reporting. Since there is no “gold” standard LAC assay, all three expert panels agree on: 1) prolongation of a phospholipid-dependent clotting assay, 2) inhibitory effect demonstrated in a mixing test, 3)demonstration of the phospholipid-dependence by a confirmatory assay, and ruling out a factor deficiency as the cause of the prolonged APTT. All three expert panels agree on and recommend that the dRVVT be used as one of the principle assays in LAC testing. However, there are some differences concerning the order of tests and the use of additional LAC tests that are worth noting. ISTH/SSC recommends the dRVVT as the first line of screening tests followed by a LAC sensitive APTT (low phospholipid concentration). BCSH and CLSI recommend the dRVVT and an APTT, however they do not state that a “low” phospholipid concentration be used in the APTT reagent. In addition, they do not exclude the use of other LAC assays such as the KCT, dPT, TTI, etc in the initial selection of screening tests as long as different coagulation pathways are tested. ISTH/SSC recommends silica as the activator in the screening APTT assay while CLSI has no restriction on the activator used. Concerning the number of specific assays to use in a LAC panel, ISTH/SSC limits the choice to the dRVVT and a LAC sensitive APTT, while CLSI places no limit on the number of assays that can be used. It is worth mentioning, however, that the use of more than two assays may increase the frequency of a false-positive result.
Determining the cut-off value for a positive LAC assay can be somewhat challenging. ISTH/SSC recommends using the 99th percentile.32 BCSH recommends validating previously established cut-offs and validating with a smaller number of normal donors (20 to 60).32 CLSI recommends determining the cut-off from the RI at +2 SD for each test in the integrated assay. 32
CONCLUSION
There remains some disagreement regarding testing strategies for LAC testing between the expert panels. There are a number of important factors to consider when performing LAC testing. These include, but are not limited to, the type of screening APTT reagent; the type(s) and number of LAC-specific assay(s) to include in the panel; whether to use normalized versus non-normalized ratios and determining the appropriate cut-offs for LAC-specific assays; and, whether to include the mixing study as a stand-alone assay or integrated into a LAC-specific assay. A thorough understanding of the pre-analytical, analytical, and post-analytical variables that interfere with LAC testing and interpretation is essential to improve testing and diagnosis. Looking to the future, the development of a single assay that could be used as an alternative to the current LAC assays that would be robust enough to recognize the heterogeneous nature of the LAC would be ideal. This assay should not be affected by the pre-analytical, analytical, and post-analytical variables previously mentioned. Finally, there is clearly a need for improved harmonization of the guidelines between the expert panels to simplify testing strategies and improve diagnostic accuracy of the assays.
- © Copyright 2016 American Society for Clinical Laboratory Science Inc. All rights reserved.