This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Dale Telgenhoff
, Oakland University, dtelgenh{at}gmail.com
INTRODUCTION
A 50-year-old male visits his general practitioner complaining of tiredness and intermittent bouts of constipation and abdominal discomfort. His stools are generally normal, and he does not report seeing blood present. He has never had a colonoscopy, even though his father died from complications due to colon cancer at the age of 64 years. His practitioner orders a take-home stool occult blood test and colonoscopy within the next month. Two of the 3 occult blood test cards returned to the laboratory test positive for blood. He schedules the colonoscopy with the referred gastroenterologist, and during the routine exam 8 polyps are removed from his rectum. The gastroenterologist sends the polyps to the anatomic pathology laboratory for examination and recommends the patient follow up with his general practitioner and that he return in 1 years’ time for a second colonoscopy. The colonoscopy report from the pathologist identifies the presence of friable and infiltrating tumor consistent with adenocarcinoma.
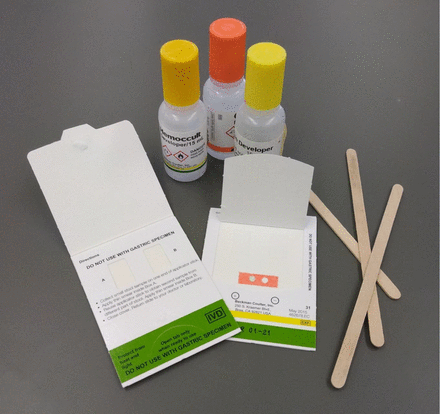
This scenario may sound familiar to you or someone in your family because colorectal cancers (CRCs) are the second most common cause of cancer death in men and women, affect around 4% of the population in their lifetime, and have an incidence estimated to be around 151 000 new cases in 2022 and a 5-year relative survival rate of around 65%.1,2 Fecal occult blood testing or fecal immunochemical-based tests are easy, noninvasive at-home tests that are useful for an initial screen, as outlined in the above scenario (Figure 1). Newer tests include the multitarget stool DNA test, which looks for both blood and markers associated with adenomas and adenocarcinomas.3 Colonoscopy is the recommended follow-up to any positives in these tests. Routine colonoscopy can be useful in catching these polyps before they progress and is recommended for those who are 45 years of age or older, or younger if there is a family history of the disease.4 Sadly, over the past 2 years, there has been a hesitancy to adhere to many routine screening programs,5 and routine colonoscopies dropped by 38.5% in one study examining pandemic-level screenings compared to the same testing centers in 2019.6 This is unfortunate because colonoscopy has been shown to be effective in reducing CRC incidence and mortality, with an estimated reduction of 38% of CRC deaths in the United States alone.7 Although we do not yet know the full impact of these pandemic-related interferences on identification and treatment, an estimated 700 cases of CRC went undetected in April of 2020 alone.6 In the case history just listed, the identification of infiltrating adenocarcinoma means there is a need to treat the patient as quickly and effectively as possible prior to invasion and metastasis.
Fecal occult blood testing (FOBT). The FOBT is an easy, noninvasive take-home test to examine blood hidden in the stool. Patient education is important to avoid false-positive or false-negative results, and positive tests should be followed up with advanced testing.
In this Focus Series, we review the role of the laboratory in patient assessment and analysis required in the diagnosis and prognosis of CRC. In the first article, “Classification and histological characteristics of colorectal cancer,” we examine the different types of colorectal carcinoma and the progression from nonneoplastic lesion to adenocarcinoma. The second article in the series, “Histologic and immunochemical assessment of colorectal cancers,” focuses on the pathology assessment used in grading and staging of the tumor as well as the laboratory tests utilized in the diagnosis and prognosis of the disease. In part 3, “Molecular characterization of colorectal cancers,” we examine the molecular testing done on the patient and the tumor in order to identify actionable targets and provide more precise treatments based on guidelines from the American Society for Clinical Pathology, College of American Pathologists, American Society of Clinical Oncology, and Association for Molecular Pathology. Finally, we discuss future directions and emerging data for expanded multigene panel testing.
- Received May 6, 2024.
- Accepted August 11, 2024.
American Society for Clinical Laboratory Science