This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Bethany N. Kent, PhD, MLS(ASCP)CM⇑
- Address for Correspondence: Bethany N. Kent, PhD, MLS(ASCP)CM, PathGroup Labs, LLC, 658 Grassmere Park, Nashville, TN 37211, 615-562-9480, bkent{at}pathgroup.com
Describe why Zika virus, Cytomegalovirus, and Mycoplasma genitalium infections are gaining attention in the study of sexually transmitted diseases.
Explain the current status of diagnostic testing for these emerging sexually transmitted infections.
Illustrate why congenital infections are linked to sexually transmitted diseases.
Abstract
Although the majority of sexually transmitted infections have been extensively studied, recent events have brought three new or emerging STDs into the public spotlight. Cytomegalovirus and Zika virus infections cause a mild flu-like illness in adults but have devastating consequences when transferred congenitally. The recent epidemic of Zika virus has led to an increase in and renewed interest in these two viral infections. Mycoplasma genitalium, on the other hand, causes severe genital symptoms but its minimal biochemical nature has made isolation and study difficult. This article will describe the prevalence, diagnosis, and treatment of Cytomegalovirus, Zika virus, and Mycoplasma genitalium.
ABBREVIATIONS: STD Sexually Transmitted Disease, CMV Cytomegalovirus, CDC Centers for Disease Control, NGU Nongonoccocal urethritis, PID Pelvic Inflammatory Disease, HCV Hepatitis C Virus RT-PCR Real-time polymerase chain reaction, RT-RPA Recombinase Polymerase Amplification Assay
INTRODUCTION
While the existence of Cytomegalovirus, Zika virus, and Mycoplasma genitalium may not be new, recent publicity has placed a considerable focus on sexual and/or congenital infection with these pathogens. A significant increase in birth defects due to congenital infection with Zika virus in South and Central America has brought interest in Zika virus transmission and treatment to the forefront of infectious disease. Likewise, the increased focus on Zika virus has seemingly renewed the public interest in the consequences of congenital infection with Cytomegalovirus. A different type of interest has become evident for infection with Mycoplasma genitalium, as it has gone from a relatively unknown cause of genital infection to being considered a substantial sexually transmitted disease.
Cytomegalovirus
Cytomegalovirus is most often know as a prevalent, mostly harmless, respiratory infection. The CDC estimates that one-in-three children under the age of five have been infected; the rate increases to >50% of adults over the age of 40.1 Like other members of the Herpes family of viruses, CMV stays latent in infected persons until a weakening of the immune system allows the virus to reactivate and one person can be infected with multiple strains of CMV.1
Transmission
CMV is an efficient transmitter as it sheds from multiple body fluids, including saliva, urine, milk, and genital. CMV can be spread through direct contact with any of these body fluids. Transmission often occurs from small children through the urine or saliva. Congenital infection can occur from an infected mother to her fetus.1
Symptoms
Cytomegalovirus (CMV) is a member of the Herpesviridae family with indications that can range from asymptomatic, mild, or a more severe mononucleosis-type disease exhibiting fever, sore throat, fatigue, and swollen glands.1 While CMV rarely causes severe complications in healthy children or adults, congenital infection with CMV has serious consequences. CMV is the most common congenital viral infection in the US. CMV causes hearing loss and neurodevelopmental delays; the long-term effects are complicated by the majority of newborns being asymptomatic (85-90%) and the health impact of infection not seen until developmental milestones are not met.2,3 For symptomatic infants, sensorineural hearing loss occurs in 35%, cognitive impairment in approximately 65%, and death in 4%.2
Diagnosis
Congenital CMV infection can be diagnosed at 2-3 weeks of age from an infant's blood, urine, or saliva.1 To assess the risk of congenital CMV infection, screening of maternal primary infection can be performed on symptomatic women, but it is not routine.4 Seroconversion of IgG or the dual presence of IgG and IgM are marks of primary infection.2 More definitive testing is focused on IgG avidity before the 16-18th week of gestation.4 Confirmatory testing is performed on amniotic fluid; a concordance between viral culture and PCR results is recommended to validate maternal infection.2
Treatment
Effective treatment of CMV has been challenging. The antiviral agents ganciclovir, valganciclovir, foscarnet, and cidofovir have all been studied for effectiveness but can have toxic side effects such as renal toxicity.5 Ganciclovir is not an option for prenatal treatment because it may be a mutagen; valaciclovir, however, does not have the same toxic properties and can be prescribed.2 The ability to be infected with multiple strains of CMV simultaneously and concurrently has hindered development of a vaccine.2
Zika Virus
Zika virus was first reported as a zoonotic pathogen infection in rhesus macaque monkeys in Uganda in 19476 and showed sporadic human infection over the next few decades.7 The first major outbreak in humans, which occurred far from its origin in Africa, was reported in Micronesia 60 years after the initial identification of the virus.8
Transmission
While the primary mode of transmission in the Americas has been linked to the Aedes aegypti mosquito9,10, the isolation of Zika virus from the semen of an infected man demonstrated a likelihood of sexual transmission as well.11 Later studies have shown that Zika virus can be detected in male semen up to 188 days after the initial onset of symptoms.12 Sexual transmission has now been confirmed to occur and is significantly more likely to occur from a symptomatic male with 96.2% of analyzed cases occurring male-to-female.12,13
Zika virus has quickly become a major concern for those who live outside of areas where the viral disease has traditionally been more prevalent. Current CDC statistics cite 5,074 total Zika cases reported in the United States and 38,306 in the US Territories,14 with the majority being transmitted by mosquito bites. However, forty-five cases in the United States are confirmed to have been transmitted sexually and twenty-eight transmitted congenitally.14,15 In November, 2016, the first case of infection from a mosquito within the continental United States was confirmed in Brownsville, TX,15 indicating the likelihood that Zika prevalence in the US may be in its infancy.
Symptoms
For the first several decades after Zika virus was discovered, it was thought to be a minor and self-limiting illness as the majority of those infected show no symptoms.16 Symptomatic patients often present with fever, headache, joint pain, rash, and conjunctivitis that lasts for up to one week.17 However, analysis of data from multiple Zika outbreaks have shown that a small percentage develop Guillain-Barre Syndrome, an autoimmune disorder that damages nerve cells and can lead to paralysis.16 Of great concern is the frequency with which Zika virus transmits from a pregnant woman to her fetus. Associations have been made between a mother with a history of Zika virus infection during pregnancy and congenital microcephaly, congenital ocular abnormalities, and stillbirth in her offspring.18,19,20 Surveillance of pregnancy outcomes by the CDC report 47 infants born with birth defects and five pregnancy losses with birth defects out of 1,143 patients reported.21
Diagnosis
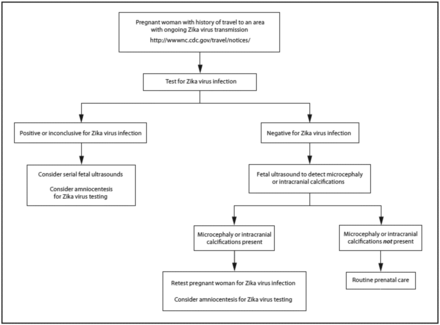
The CDC recommends that only patients who meet the specific Clinical Guidance Guidelines undergo diagnostic testing for Zika virus (Figure 1). Two methodologies are available for diagnosis of Zika virus: RT-PCR and serology via and IgM ELISA. There are currently no FDA-approved diagnostic tests for the Zika virus. As Zika virus infection becomes an increasingly visible concern to US citizens, the FDA has granted Emergency Use Authorization to several diagnostic assays.
While the majority of Zika testing is real-time reverse transcriptase (RT)-PCR, a recent publication has shown promising results for a point-of-care Zika test utilizing a portable reverse transcription isothermal recombinase polymerase amplification assay (RT-RPA) that can yield results in 15 minutes using a urine sample.22 A multiplex assay that can quickly distinguish between Zika virus and Dengue virus is in development.23
Treatment
Although the FDA has not yet approved any drug for the treatment of Zika virus, preliminary studies have shown that several drugs approved for treatment of other diseases have shown efficacy against Zika virus.24 The most promising candidates are emerging as those drugs used to treat other Flavivirus infections, such as Hepatitis C virus (HCV). The drug sofosbuvir, which is approved for treatment of HCV, can inhibit replication of Zika virus in cell lines by binding to amino acids in the Zika virus RNA polymerase that are used for ribonucleotide incorporation.25 Another treatment for HCV, the viral polymerase inhibitor 7-deaza-2’-C-methyladenosine (7DMA), inhibits viral replication to the point of decreased mortality in mice.26 The malaria drug chloroquine has also shown antiviral capabilities,27 as have 20 other drugs, such as daptomycin and bortezomib, tested in a large in vitro screen.28
The CDC Recommended Chart for Zika virus testing.21
Mycoplasma genitalium
Mycoplasma genitalium was first identified as the possible cause of sexually transmitted infection in the late 1970s when men undergoing treatment for nongonococcal urethritis (NGU) were cleared of infection with tetracycline despite what appeared to be a negative culture.29,30 A multitude of subsequent studies have confirmed the association between M. genitalium and NGU symptoms,29 which has led to the study of M. genitalium's role in sexually transmitted infections.
Transmission
Evidence for sexual transmission of M. genitalium is based on the correlation of presence of the organism and symptoms of urogenital infection and the preference of M. genitalium to colonize the human urogenital tract.29
Symptoms
M. genitalium is suspected to cause 20-35% of male NGU not caused by chlamydia.31 In men, M. genitalium has been linked to inflammation of the glans penis and prepuce (known as balanoposthitis), chronic prostatitis, and acute epididymitis. In women, it has been associated with urethritis, bacterial vaginosis, cervicitis, and pelvic inflammatory disease.29,32 Studies have shown associations between M. genitalium and other sexually transmitted infections including HIV and HPV.29,33
Treatment
Doxycycline was the original treatment of choice for NGU; however, it is ineffective against a significant number of isolates.34 Treatment failures led to the prevalence of azithromycin or moxifloxacin as the primary treatment options but recent studies have identified mutations in M. genitalium rendering the organism resistant to these antimicrobials. 34-37 Much like the threat of antibiotic resistant gonorrhea, the identification of multiple resistance-causing mutations in M. genitalium could lead to untreatable NGU.38
Diagnosis
M. genitalium is the smallest free-living organism and this evolution to a minimal genome state means that direct culturing from patient specimens requires cell culture and is prone to failure,39-41 including by cross-contamination.29 When culture is successful, it can take up to six months for isolates to grow.38 Nucleic acid tests are widely considered the most accurate and efficient for definitive diagnosis of the presence of M. genitalium; however, there are currently no FDA-cleared tests available.38
CONCLUSION
Cytomegalovirus and Zika virus arose from different viral families but cause similar symptoms in primary infections. They both have been shown to have multiple modes of transmission in addition to sexual transmission but are a major concern due to their link to severe congenital developmental issues. The incredible amount of research going into understanding Zika virus infection, transmission, and treatment will lead to huge advancements in understanding congenital viral infections of these types. On the bacterial spectrum of emerging sexually transmitted diseases, understanding of Mycoplasma genitalium has been slow due to sheer difficulty in isolation and cultivation. The link of M. genitalium to a high number of NGU infections and its ability to demonstrate antibiotic resistance has solidified the need for further study.
- © Copyright 2017 American Society for Clinical Laboratory Science Inc. All rights reserved.