This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Sara Taylor
, Tarleton State University, sataylor{at}tarleton.edu
ABSTRACT
OBJECTIVE: This case study outlines the diagnostic schema employed at our facility to accurately identify a patient’s hematological condition to facilitate optimal treatment.
BACKGROUND: A 71-year-old male patient presented to our hospital with complete blood count data that were suggestive of a lymphoid malignancy due to a critically low leukocyte count coupled with a predominance of lymphocytes displaying abnormal morphology.
METHOD: Immunophenotyping studies carried out on the bone marrow indicated that the patient had a B lymphocyte malignancy, although a T-cell neoplasm could not be ruled out. Karyotyping on this patient revealed a complex karyotype, which supported the conclusion that this patient suffered from a malignant condition. Finally, molecular diagnostic testing was employed to specifically identify the disease state.
CONCLUSION: Although the testing results are suggestive of a lymphoid malignancy, the patient’s diagnosis remains unclearly defined.
- GERD - Gastroesophageal reflux disease
- CBC - complete blood count
- CCND1 - cyclin D1
- CLL/SLL - chronic lymphocytic leukemia/small cell lymphocytic leukemia
- DLBCL - diffuse large B-cell lymphoma
- FISH - fluorescence in situ hybridization
- FL - follicular lymphoma
- GERD - gastroesophageal reflux disease
- HCL - hairy cell leukemia
- IgH - immunoglobulin heavy chain locus
- JAK2 - Janus kinase 2
- MALT - mucus-associated lymphoid tissue
- MCL - mantle cell lymphoma
- NK - natural killer
- PCR - polymerase chain reaction
- sIg - surface immunoglobulin
- TRB - T-cell receptor beta
- TRD - T-cell receptor delta
- TRG - T-cell receptor gamma
- WBC - white blood cell
INTRODUCTION
In this case history, a patient has presented to our facility with severe pancytopenia. The few white blood cells (WBCs) present in his peripheral blood were mostly lymphocytes, many of which possessed markedly aberrant morphology. In presenting the patient’s laboratory testing results, we outline the diagnostic schema employed at our facility in an attempt to accurately identify the patient’s disorder so that optimal treatment could be administered.
CASE STUDY
History
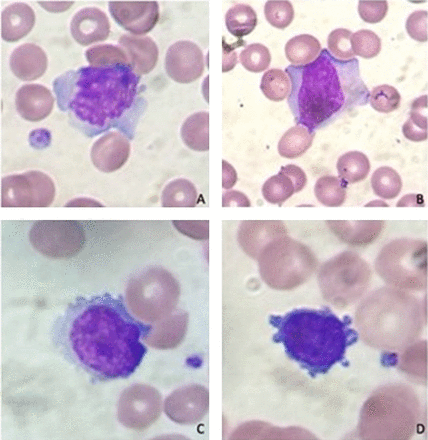
We report a case of a 71-year-old man who presented at the emergency department complaining of generalized weakness, lightheadedness, and an approximate weight loss of 20 pounds, all occurring during the preceding 4–6-week period. His physical examination was mostly unremarkable, but his past medical history included depression, chronic back pain, and gastroesophageal reflux disease (GERD). A complete blood count (CBC) revealed severe pancytopenia including a WBC count of only 800/μL. His hematocrit and platelet count were low as well, 21.1% and 57,000/μL, respectively. A manual differential revealed a predominance of lymphocytes (76.7%) and granulocytopenia (16.3%) (Table 1). Morphologically, the lymphocyte population was determined to include atypical lymphocytes (Figure 1A–D), but the other cell lines were assessed as normal, with no signs of pathology or immaturity. To investigate the cause of the patient’s pancytopenia, a bone marrow aspiration and biopsy were performed. While the clot section was notably hypocellular with poorly identified bone marrow elements, a single aggregate of bone marrow tissue displayed 90% cellularity of monomorphic cells. The biopsy specimen revealed extensive replacement of the normal bone marrow with reticulin and collagen fibers. A few scattered normal marrow cells were seen in the background, along with fibroblasts, and larger cells that were possibly blast cells but could not be further evaluated in the biopsy specimen.
Patient’s pertinent laboratory results
Atypical lymphocytes in patient’s peripheral blood. (A, B) Reactive appearing lymphocytes. (C, D) Lymphocytes with cytoplasmic extensions.
Further Testing
To rule out a lymphoma and/or leukemia, karyotyping, immunophenotyping, and several molecular diagnostic tests were performed on the bone marrow specimen. Cytogenetic analysis revealed an abnormal male karyotype:
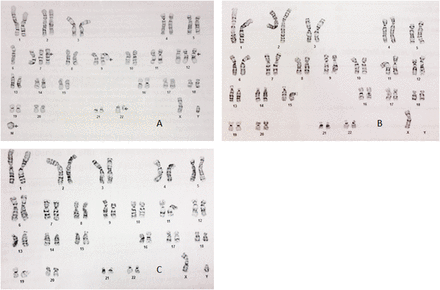
48,XY,−6,+7,t(9;22)(p24;q11.2),+12,add(14)(q24),+r[3]/45,X, −Y[8]/46,XY[9] (Figure 2A–C). Flow cytometric analysis of the bone marrow showed few blasts (0.3%), significantly decreased neutrophils (9%) with no significant marker abnormalities, 4% monocytes normal for markers tested, and normal eosinophils/basophils. T lymphocytes were immunophenotypically normal although they were increased in number and present in an abnormally low CD4:CD8 ratio (1.3, NR = 2.0). The remaining cells were assessed as natural killer (NK) lymphs (normal in numbers and markers) and B lymphocytes. Eight percent of the cells assessed as B lymphocytes were immunophenotyped as CD45+, CD19+, CD20+, CD5−, CD10−, CD23−, FMC7+, CD38−, CD11c+, CD103−, HLA DR+, and surface immunoglobulin (sIg) kappa+. To help determine if the patient suffered from mantle cell lymphoma (MCL), follicular lymphoma (FL), or mucus-associated lymphoid tissue (MALT) lymphoma, DNA probes for BCL1 (t(11;14), B-cell lymphoma 2 (BCL2) (t(14;18), and MALT1 rearrangements were utilized in fluorescence in situ hybridization (FISH) testing. The patient was found to display normal results in each case. T-cell receptor (TCR) gene rearrangement analysis by polymerase chain reaction (PCR) was used to examine clonal T cell receptor gene rearrangements. Specifically, the detection parameters included T-cell receptor gamma (TRG V1-8, 9 + J1/2), TRG alternate V + J1/2, T-cell receptor beta (TRB V + J1/2), TRB V _ J2, and TRB D + J1/2. Only the TRB D + J1/2 gene rearrangement could be detected (Table 2). In addition to hematology and molecular diagnostic testing, a fungal serological panel that included testing for Blastomyces, Coccidiodes, Histoplasma, Aspergillus species was performed and found to be negative. The patient tested nonreactive for HIV-1/HIV-2 and negative as well for tuberculosis.
Patient’s karyotype: 48,XY,−6,+7,t(9;22)(p24;q11.2),+12,add(14)(q24),+r[3]/45,X,−Y[8]/46,XY[9]. (A) Cytogenetic analysis revealed three of 20 metaphase cells with loss of one copy of chromosome 6, a gain of a copy of chromosomes 7 and 12, a reciprocal translocation involving the short arm of chromosome 9 and the long arm of chromosome 22, additional material of unknown origin on the long arm of chromosome 14, and gain of an unidentifiable ring chromosome. (B) Eight cells showed loss of the Y chromosome. (C) Remaining cells were apparently normal.
Flow cytometry and molecular testing
DIAGNOSIS/DISCUSSION
Some of the lymphocytes of this patient’s bone marrow had an abnormal appearance and displayed an immunophenotype that was suggestive of various B-cell leukemias/lymphomas. The negative CD5 and CD10 immunophenotype supports speculation that the patient has developed either hairy cell leukemia (HCL) or a lymphoplasmacytic lymphoma. A hematology consult resulted in a suggestion that HCL might be considered based on the appearance of the peripheral blood lymphocytes, many of which had cytoplasmic extensions characteristic of HCL (Figure 1C and 1D). However, the immunophenotype was ambiguous for HCL since the cells were negative for CD103. The panel did not include testing for CD25 and CD123, both of which are expected to be positive in HCL. If HCL was strongly suspected, BRAF V600E mutation testing could have helped provide a differential diagnosis since the mutation exists in a majority of HCL patients.1,2
The CD5− and CD10− immunophenotype also suggests the possibility that the pathological condition is a lymphoplasmacytic lymphoma. A positive outcome for the MYD88 L265P mutation would have confirmed such a putative diagnosis, but the patient’s clinical and laboratory presentation did not indicate that this presumptive diagnosis should be pursued.3,4
Cytogenetic analysis revealed multiple numerical and structural abnormalities including monosomy 6, trisomy 7 and 12, a reciprocal translocation of the short arm of chromosome 9 and the long arm of chromosome 22, additional material of unknown origin on 14q, and an unidentifiable ring chromosome (Figure 2A). Eight of 20 cells showed a loss of the Y chromosome (Figure 2B; Table 2). The finding of multiple abnormalities in the cytogenetic analysis report indicates a neoplastic process. The correlation of peripheral T-cell malignancies with loss of heterozygosity of chromosome 6 suggests that chromosome 6 houses tumor suppressor cells, most likely on the long arm.5,6 Trisomy 7, trisomy 12, and aberrations of 14q all may be observed in B-cell lymphoproliferative disorders. The t(9;22) translocation at p24;q11.2 results in the fusion of Janus kinase 2 (JAK2) to breakpoint cluster region (BCR) and leads to constitutive activation of JAK2 kinase. Subsequent neoplastic transformation and abnormal cell proliferation in both myeloid and lymphoid malignancies may follow.7,8 Loss of the Y chromosome is common in hematopoietic cells of older males and is considered to be an age-related phenomenon with no known clinical significance when seen in the relatively moderate number of cells displayed by this patient.
FISH target gene analysis was performed to identify translocation/fusion genes using IGH/BCL1 and IGH/BCL2 DNA probes to rule out MCL and FL. Negative results for this analysis showed that neither the cyclin D1 (CCND1) gene nor the BCL2 gene were juxtaposed with the immunoglobulin heavy chain locus (IgH) promoter region on chromosome 14 as is expected in MCL and FL.9,10 The previous negative results for both CD5 and CD10 suggested that these lymphomas were not likely candidates; however, since the patient’s immunophenotype results provide unclear information about this patient’s pathology, investigating these lymphomas was reasonable. MALT was another possible diagnosis, although once again the immunophenotype was ambiguous. To investigate the possibility of a MALT lymphoma, a MALT1 break-apart probe was used to interrogate the presence of the most commonly occurring translocation seen in MALT, the apoptosis inhibitor 2:mucous associated lymphoid tissue 1 gene fusion.11 The test results for the FISH analysis were all within the normal reference range and did not reveal any abnormality (Table 2).
At this point, Burkitt lymphoma, diffuse large B-cell lymphoma (DLBCL), and chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL) might have been considered as a diagnosis for the patient despite ambiguous immunophenotyping results, but the decision about which possibilities to explore must be made within the context of the clinical and pathological information presented. The patient’s clinical presentation, CBC, and immunophenotype were not predictive of these B-cell neoplasms; therefore, testing directed towards identifying these pathologies was not pursued.
Molecular clonality assays investigating immunoglobulin (Ig) or T-cell receptor rearrangements are sometimes useful in diagnosing suspect T- and B-cell malignancies when the morphology and immunophenotyping are inconclusive. In humans, the T-cell receptor is composed of two different protein chains; α, β, δ, or γ, encoded by TRA, TRB, TRD, and TRG. Since lymphomas and leukemias are derived from a single malignantly transformed lymphoid cell, the tumor cells of T-cell malignancies should contain identical clonal gene rearrangements. Heterogeneity in TCR rearrangements identifies the presence of cells activated by a nonmalignant reactive condition.12,13 Theoretically, TRA, TRB, TRD, and TRG gene rearrangement can be examined; however, commonly only TRG and TRB rearrangements are interrogated since most T-cell malignancies exhibit rearrangements in these genes. TRG genes are a classic clonality target since rearrangement of this gene occurs early in T lymph development; TRB rearrangements are very useful for assessing T-cell malignancies since rearrangement of this gene occurs in almost all mature T-cell malignancies.14 TRG V1-8, 9 + J1/2, TRG alternate V + J1/2, TRB V + J1/2, TRB V _ J2, and TRB D + J1/2 were all examined; only the TRB D + J1/2 gene rearrangement could be detected (Table 2). Assessment of gene rearrangement has been greatly standardized in recent years, but interpretation can still be difficult. Certainly, there are many discrepancies involving DNA quality, tissue representation, and in expertise/knowledge of clonality issues that can make interpretation ambiguous. In this case, the presence of only one PCR product among the different parameters that were tested most likely means that the sole positive result is due to something other than clonal gene rearrangement. Some lymphomas are only revealed by beta and not gamma rearrangement, but it is expected that some of the other beta rearrangements would be positive as well. The likely explanation for this particular result is that it was due to artifact, which could include polyclonal T cells.
Treatment
For this patient, a definitive diagnosis remains elusive. Without question, there are multiple abnormal findings among the patient’s results, some of which indicate a neoplastic process of a lymphoid nature. After consultation with the patient, a decision was made that conservative management is appropriate for now. Thus, the patient has been advised to monitor blood pressure, follow a healthy diet, control his anemia, and control hydration. The patient was discharged after receiving a dose of filgrastim to increase his low neutrophil count.
- Received February 18, 2018.
- Accepted February 18, 2018.
American Society for Clinical Laboratory Science