This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Department of Immunology and Microbiology, School of Medicine, Anschutz Medical Campus, University of Colorado
- Department of Honors Studies, Texas Tech University
- Department of Honors Studies, Texas Tech University
- Department of Honors Studies, Texas Tech University
- Address for Correspondence: Allie Clinton Smith
, Department of Honors Studies, Texas Tech University, allie.c.smith{at}ttu.edu
ABSTRACT
A burdensome, atypical phenotype of Staphylococcus aureus (SA) called SA small colony variants (SCVs) has been identified, which is induced because of a combination of environmental stressors, including polymicrobial interactions. SA-SCVs exhibit altered phenotypes because of metabolic dormancy caused by electron-transport deficiency, which leads to increased biofilm production and alterations to antimicrobial susceptibility. SA-SCVs typically exhibit altered colony morphology and biochemical reactions compared with wild-type SA, making them difficult to detect via routine diagnostic procedures. SA-SCVs have been found to contribute to chronic or recurrent infections, including skin and soft-tissue infections, foreign-body–associated infection, cystic fibrosis, and sepsis. There is evidence that SA-SCVs contribute to patient morbidity and mortality rates because of diagnostic difficulties and limited treatment options. New detection methods may need to be developed that can be incorporated into routine diagnostic procedures, which would allow for better assessment of specimens and introduce new considerations for management.
- AST - antimicrobial-susceptibility testing
- ATP - adenosine triphosphate
- CF - cystic fibrosis
- CHOC - chocolate agar
- eap - extracellular-adherence protein
- ETC - electron-transport chain
- HQNO - 2-heptyl-4-hydroxyquinoline-N-oxide
- MALDI-TOF - matrix-assisted laser desorption/ionization time of flight
- MIC - minimum inhibitory concentration
- MS - mass spectrometry
- PA - Pseudomonas aeruginosa
- PCR - polymerase chain reaction
- PJI - prosthetic-joint infection
- SA - Staphylococcus aureus
- SAIDE - CHROMID S. aureus Elite agar
- SCV - small colony variant
- SMX - sulfamethoxazole
- SSTI - skin and soft-tissue infection
- TMP - trimethoprim
- WT - wild type
INTRODUCTION
Staphylococcus aureus (SA) is a bacterial species often found as a transient colonizer on the human body, particularly as part of the nasal microflora in approximately 30% of the population.1⇓-3 Despite this, SA will often act as an opportunistic pathogen that secretes multiple virulence factors within its host and interacts with other pathogens, ultimately contributing to a wide range of human infections, both acute and chronic.2,4⇓-6 SA infections are important contributors to chronic infections, which are difficult to diagnose and treat because of antimicrobial resistance—including multidrug resistance—and the polymicrobial nature of chronic infections.7⇓⇓-10
A burdensome, atypical phenotype of SA, called SA small colony variants (SCVs), has been identified as largely associated with chronic and recurrent infections, such as cystic fibrosis (CF), foreign-body–associated infections, skin and soft-tissue infections (SSTIs), and sepsis.6,7,11⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-23 This phenotype contributes to recurrent infection via increased biofilm formation, altered antimicrobial susceptibility, and intracellular persistence to evade the host’s defense mechanisms.5,8,9,11,22,24⇓-26 Specific nutritional requirements, called auxotrophy, of chemicals hemin, menadione, and thymidine cause SA-SCVs to be unable to produce key components for a functional electron-transport chain (ETC), which leads to a deficiency in electron transport and characterizes the phenotype as metabolically dormant.5,6,24,25,27,28 Consequently, anoxic conditions increase SA biofilm production and alter antimicrobial susceptibility, rendering the infection more difficult to treat.5,8,14,22,24,28,29
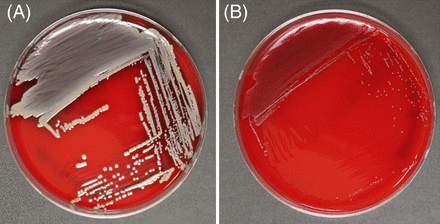
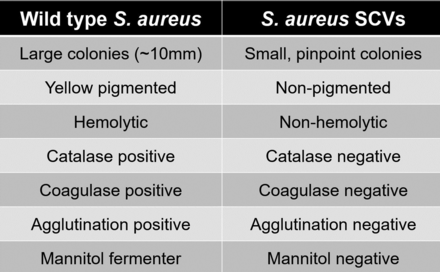
SA-SCVs are distinguished from wild-type (WT) SA by their unusual morphological characteristics and biochemical reactions.5,11,28 SA-SCVs are characterized by small, pinpoint colony sizes almost 10 times smaller than WT-SA colonies (Figure 1).5,6,11,22,28,30 SA-SCVs exhibit a slow growth rate, decreased pigmentation, and decreased hemolysis.5,6,14,22,31 Because of slower growth rates, SA-SCVs require a longer incubation time (approximately 48–72 hours for optimal growth) and are often overgrown by WT-SA and other organisms of interest in coculture, which makes coculture detection of SA-SCVs difficult.5,11,14 This phenotype is spontaneously induced by environmental stressors, such as harsh conditions or polymicrobial interactions.25,26,32⇓-34 This phenotype may be unstable and revert to normal growth, WT-SA colony morphology, and WT-SA biochemical responses.5,9,22,25,28,30 Ultimately, these morphological characteristics can lead to misidentification or identification failure (Figure 2).5,11,25,26
Morphological differences exhibited by WT-SA and SA-SCV. Blood agar plates after 48 hours of incubation at 37ºC, showing a comparison of the colony morphology of (A) WT-SA to (B) SA-SCV phenotype. WT-SA with the normal phenotype, characterized by yellow pigmentation and hemolysis (A, SA Newman parental strain54). SA-SCV, characterized by pinpoint colonies that are nonpigmented and nonhemolytic (B, SA NewmanΔmenB55). Images taken by Klara C. Keim; strains provided by Catherine Wakeman, Texas Tech University Department of Biological Sciences.
Morphological and biochemical characteristics distinguishing WT-SA from SA-SCVs.
The major clinical implication of the SA-SCV phenotype is chronic or recurrent infections, which often contributes to patient mortality rates because clinical microbiologists are often unable to detect, identify, and effectively treat the SA-SCV infection.5,8,24,28,31 Currently, there is no routine diagnostic procedure in the clinical laboratory for detecting the SA-SCV phenotype.24,31,35,36 Many clinical laboratories are unfamiliar with this phenotype, which can often lead to oversight of these small colonies or misidentification as commensal organisms.11,21,25,37 Altered antimicrobial susceptibility, abnormal biochemical test results, and morphological appearances collectively leave SA-SCVs frequently misidentified or undetected, limiting treatment options and success of the infection.5,11,31,35,36 This stresses the importance of familiarizing the clinical laboratory with the morphological and biochemical characteristics, mechanisms of induction, implications of SA-SCVs in clinical practice, and new detection methods into routine procedure. Ultimately, this will lead to better potential SA-SCV infection specimen assessment and introduction of new treatment options.
INFECTIOUS PROCESSES
Most patient culture SA-SCV isolates are significant causative agents of recurring and persistent infections, and they lead to clinical implications of infection progression caused by detection difficulties and poor antimicrobial therapy response.5,6,14,28 SA-SCVs optimize their capacity to act as a reservoir for chronic infection by expressing altered patterns of virulence factors, increased biofilm formation, and intracellular persistence in host cells.9,19,28,35 SA-SCVs have been isolated in chronic diseases and infections, such as CF, osteomyelitis, persistent SSTIs, chronic wounds, foreign-body–associated infections, and sepsis.5,6,11,13,22 Increased hospital intensive care unit mortality rates, prior exposure, long-term antimicrobial therapy, minimum inhibitory concentration (MIC) alterations, and treatment failure have been reported for infections harboring SA-SCVs compared with WT-SA.11 Some SA-SCV infections have persisted asymptomatically for years posttreatment and have exhibited recurrent relapse.9 Therefore, SA-SCVs may not be rare in infection progression but are often difficult to recover in the clinical laboratory because of previous misidentification or nonidentification as causative agents in chronic infection.11
Cystic Fibrosis
The most frequently studied infectious process associated with SA-SCVs is in patients with CF. Clinical studies have found that approximately two-thirds of patients with CF exhibit coinfection of SA—one-half being SA-SCVs—and Pseudomonas aeruginosa (PA).5,11,13 Coinfection with PA can induce SA-SCVs via exotoxins within 6 hours and cause SA persistence in CF patients; SA-SCV induction may be a cumulative and specific response to the environment of the CF airways and aminoglycoside treatment30,38,39,41 SA and PA contribute independently and additively to the severity of infection in patients with CF by leading to variable SA-SCV phenotype antimicrobial resistances, potential nonidentification of SA during antimicrobial-susceptibility testing (AST), and increased lung inflammation and damage.10,13,30,34,44 In addition to significantly higher SA-SCV antibiotic resistance rates compared with WT-SA, higher proportions of methicillin-resistant SA have been reported in SA-SCV isolates than in WT-SA isolates.10,44
Patients with CF require frequent CF-related intravenous antibiotic therapy and often have received prior and long-term antibiotic treatment.44 Prior and long-term antibiotic use is a risk factor for developing SA-SCV infections, which exhibit higher MICs because of their selection by antimicrobials, specifically interventional aminoglycosides and trimethoprim (TMP)-sulfamethoxazole (SMX) therapy even after extended therapy discontinuation.10,44 Patients with CF who harbor SA infections were found to have significant resistance to SMX, TMP, gentamicin, fosfomycin, ciprofloxacin, erythromycin, and clindamycin.13 Antibiotic resistance acquired by SA-SCVs allows them to then persist and infect host cells of patients with CF for up to 50 years, often without detection.36
SA-SCV infection is directly related to poor clinical outcomes in patients with CF by contributing to chronic inflammation of lung tissue. The inflammation ultimately leads to collateral tissue damage caused by increased concentrations of host neutrophils.36,44 The combined frequency of SA and PA indicates that clinical laboratories should be encouraged to improve active efforts of detection and monitoring of SA-SCV prevalence.36
Prosthetic-Joint and Device-Related Infections
Implant-related infections harboring SA-SCVs—specifically prosthetic-joint infections (PJIs), medical-device–related infections, and prosthetic-valve endocarditis—have a poor clinical response to prolonged antimicrobial therapy, have an increased antimicrobial resistance upon implant adherence, and are identified as causative agents in recurrent infection.5,6,22 WT-SA readily attaches to medical devices and prostheses during implantation because of contamination by patient skin, medical staff, or even airborne particles.5 Host body fluids coat the surfaces, and upregulation of SA-SCV mutated toxin gene extracellular-adherence protein (eap) and adhesion-fibronectin–binding protein as well as the downregulation of α-toxin and proteinase virulence factors enhance bacteria adherence to the implant and invasion of host cells, often forming a biofilm.11,29 SA-SCV foreign-body infections can quickly lead to sepsis and bacteremia prior to isolation of the SA-SCV phenotype. Treatment of recurrent infections requires removal of all infected prosthesis, tissue, and foreign material followed by antibiotic treatment continued through reimplantation to avoid treatment failure and persistent infection.22 Therefore, clinical detection and treatment of PJIs harboring SA-SCVs must be rigorous and thorough upon initial infection to avoid treatment failure and reimplantation.22
Osteomyelitis
Osteomyelitis is a common comorbidity of device-related infections, PJIs, chronic wounds, and SSTIs caused by the spread of bacteria, which makes locating bacterial load and treatment of chronic infection difficult.37,45 SA infection is a significant causative agent of osteomyelitis, and cases harboring SA-SCVs contribute to chronic infection via osteoblast intracellular persistence.5,37,45 Bone and vascular tissue damage, which result from chronic bone infection, prolong SA-SCV persistence in poor perfusion areas because SA-SCV reservoirs cannot be reached by effective antibiotic concentrations.37,45 SA-SCV–biofilm formation is more likely in osteomyelitis infections than other SA-SCV–related chronic infections because of increased polysaccharide-intracellular–adhesin production for bone-matrix adhesion.24,37
Immediate host-cell treatment with a variety of antibiotics has been shown to reduce intracellular loads of SA-SCVs. Findings suggest that antimicrobial administration after 12 hours of intracellular persistence increases susceptibility with MICs up to 32-fold higher, and—after 7 days of persistence—antibiotic therapy became altogether ineffective.24,37,46 Rifampicin was the only treatment method to have an effect on established SA-SCV persistence within bone tissue, indicating that a sum of SA-SCV–evasion mechanisms and environmental characteristics impede the effect of other antibiotics.45 Chronic osteomyelitis SA-SCV–persistence treatment must be a combination of interventional methods to clear infection, including surgical intervention and serial debridement of infected areas with long-term antimicrobial therapy.24,37,45
Chronic Wounds and Infections of the Skin and Soft Tissue
Most conducted studies sought to identify SA-SCVs harbored in chronic infections, specifically CF, osteomyelitis, PJIs, and device-related infections. SA-SCV occurrence is prevalent in other cases of chronic infection as well, such as SSTIs, chronic wound infections, diabetic foot ulcers, and cases of sepsis. SA is the most prevalent pathogen that causes SSTIs and chronic wound infections; however, these cases have not been studied as thoroughly despite the similar observed mechanisms of SA-SCV pathogenesis and induction. WT-SA commonly acts as an opportunistic pathogen alongside other pathogens, and treatment could ultimately select for SA-SCVs in chronic wounds and present other patient burdens, specifically manifestation of diabetic foot ulcer infection in patients with diabetes.15,16,18,51,52
SA-SCVs have been isolated from patients with SSTIs who harbor chronic, polymicrobial-staphylococcal infections and who were previously administered long-term antibiotic treatment. Interestingly, in the case of SSTIs, WT-SA and SA-SCVs have been isolated in the infected tissue and within the nares and mucosae of patients, sometimes without exhibiting a clinical presence until an opportunity arises for pathogenesis.15 Notably, it has been found that SA-SCVs invade endothelial and epithelial cells of the integument and persist intracellularly, affecting the structure and integrity of the integument. SA-SCVs can also exhibit antibiotic resistance (methicillin, vancomycin, etc), cause recurrent purulent infection, limit treatment options, and infiltrate circulation to infect other organs and cause septicemia.15,35,47⇓⇓-50 SA-SCVs notably persist intracellularly within keratinocytes with low virulence as a mechanism of evading the host immune system.47 This highlights the mechanism by which SA-SCVs can persist, demonstrates decreased antimicrobial susceptibility, and allows SSTIs to recur even after the infection is thought to be eradicated. Accurate identification and effective SA-SCV treatment application are also notably important to prevent further resistance and limit patient morbidity and mortality rates in SSTI cases.15
MECHANISMS OF INDUCTION
Chronic SA infections face harsh conditions and environmental stressors, notably aminoglycoside treatment and coinfection with PA that induces an anoxic state, interfering with ETC function, and causes selective pressure for the SA-SCV phenotype.11,24,25,28,30,32,33,38⇓⇓-41 SA-SCV phenotype can be burdensome and increase biofilm formation and decrease aminoglycoside sensitivity.32,34,35,38,39,41
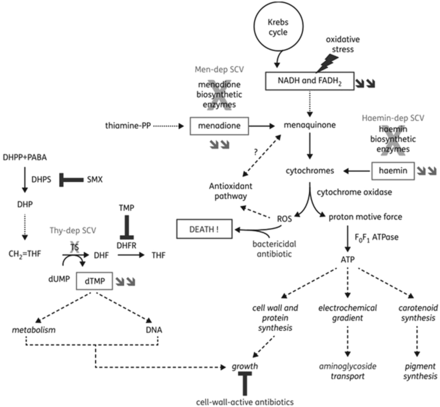
Prior exposure or long-term treatment antimicrobials, specifically subinhibitory concentrations, select for SA-SCV phenotype and decrease antimicrobial susceptibility; most notably, aminoglycosides were found to induce SA-SCV emergence and exhibit the greatest susceptibility reduction.13,25,28,29,39,41 Aminoglycosides cause mutations in genes involved in the ETC and adenosine triphosphate (ATP) synthesis in addition to biosynthesis of thymidine, menadione, and hemin.5,11,14,25,26,31 Electron-transport interference by aminoglycosides limits nutrient availability, suppresses aerobic metabolism, and causes electron deficiency that ultimately leads to spontaneous mutations that respond to selective environmental pressures. Additionally, ETC deficiency and polysaccharide-intracellular–adhesin upregulation can increase resistance and biofilm production.5,24,25,33,38⇓-40 Patients can initially harbor both WT-SA and SA-SCV strains but, following antimicrobial chemotherapy, eliminate WT-SA strains to induce and harbor predominantly persistent SA-SCVs.13,14,25,28 This leads clinicians to believe treatment has succeeded because of SA-SCV nonidentification, which can then exhibit increased resistance to aminoglycosides and contribute to persistent and recurrent infections (Figure 3).26,35
Illustration of the mechanisms leading to the SCV phenotype in SA and of their link to reduction in susceptibility to specific antibiotic classes. Double arrows refer to metabolites whose concentrations are reduced in the corresponding SCVs. Electron-transport–deficient SCVs show alterations in the pathways that lead to the synthesis of menadione or hemin (subsequent to mutations in biosynthetic enzymes), which cause a reduction in the amount of ATP produced. This leads to a reduced growth rate, which may affect the effectiveness of antibiotics active against dividing bacteria—such as cell-wall–active agents—and to a reduction in transmembrane potential, which impairs aminoglycoside uptake. Menadione-dependent SCVs are hypersusceptible to oxidant species, possibly because of reduced electron transport and alteration of the induction of antioxidant pathways (shown to be regulated by menaquinone in gram-negative bacteria). Thymidine-dependent SCVs are unable to convert deoxyuridine monophosphate to deoxythymidine monophosphate (dTMP) (using dihydrofolate [DHF] as a cofactor) because of mutations in thymidylate synthase, leading to dTMP depletion. These strains are nonsusceptible to antifolate agents that act on successive steps in this pathway, namely sulphonamides and diaminopyridines. Sulphonamides—such as SMX—are inhibitors of dihydropteroate (DHP) synthase that produce DHP from dihydropteridine pyrophosphate and para-aminobenzoic acid. Diaminopyridines—such as TMP—are inhibitors of DHF reductase, which catalyze the reduction of DHF to tetrahydrofolate. They also show a reduced growth rate. Globally, antibiotics may also be less bactericidal toward electron-transport–deficient SCVs because of a reduced production of reactive oxygen species. DHF, dihydrofolate; DHP, dihydropteroate; dTMP, deoxythymidine monophosphate; Haemin-dep, hemin dependent; Men-dep, menadione dependent; Thy-dep, thymidine dependent. Reprinted with permission from Garcia et al, 2013.24
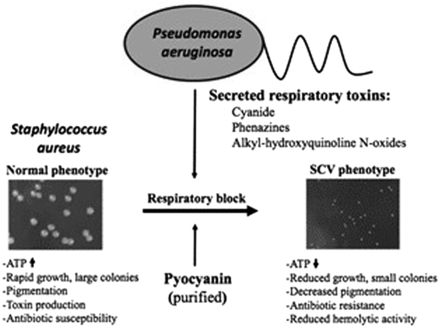
In conjunction with environmental stress induced by aminoglycoside exposure, polymicrobial interactions also contribute to emergence of the SA-SCV phenotype.30,32,34,38,40,41 SA infections, specifically those found harboring SA-SCVs, consistently exhibit coinfection with PA, leading to mechanisms of microbial competition.30,34,38 PA is often a dominant pathogen and expresses exotoxins, such as alkyl-hydroxyquinoline N-oxides (2-heptyl-4-hydroxyquinoline-N-oxides [HQNOs]), hydrogen cyanide, and pyocyanin, to act against commensal organisms in the host.28,30,32⇓-34,38,40,41 SA is sensitive to PA exotoxins like those targeting ETC and suppressing aerobic metabolism. WT-SA growth ultimately induces the electron-deficient SA-SCV phenotype that provides additional protection and resistance from aminoglycosides and vancomycin because of slowed metabolic activity (Figure 4).30,32,33,40,41 By suppressing aerobic metabolism through interference of the SA ETC, PA selects for SA-SCVs and increased biofilm formation of this phenotype as a mechanism to tolerate the compounds produced by PA.28,30,38,40 Therefore, the PA exotoxins drive SA to grow in a metabolically dormant and fermentative state as a survival strategy to persist in the presence of PA.28,32⇓-34 This indicates that aggressive and prolonged combination therapy may be required to eradicate SA-SCVs in coinfection.5,24 Additionally, it may be important for the clinical laboratory to actively screen for SA-SCVs in persistent infections detecting coinfection of SA and PA.22,35,42
Illustration of PA-induced SA-SCV selection. (Left) Colony size of SA under normal conditions. (Right) In the presence of respiratory toxins like HQNO or the Pseudomonas quinolone signal, pyocyanin or cyanide produced by PA leads to selection of the electron-transport–deficient SCV phenotype in SA. Reprinted with permission from Biswas et al, 2009.40
METHODS OF DETECTION
SA-SCVs are persistent, burdensome, and difficult-to-treat medically important organisms, and they should be actively investigated and considered differently from WT-SA by clinical laboratories.22 SA-SCVs often remain undetected or misdiagnosed in the clinical laboratory because they are a condition-, media-, and time-dependent phenotype with low metabolism and slow growth, only to be detected usually after 48–72 hours of incubation; prolonging the culture period can double detection and increase WT-SA overgrowth likelihood.5,8,25,28 The current absence of a standardized diagnostic procedure for SA-SCVs in the clinical laboratory calls for its need in reducing patient morbidity and mortality rates.
Many methods of detection have successfully cultured and detected SA-SCVs from clinical samples of chronic infection, distinguished SA-SCVs from WT-SA and other commensal organisms, and further identified antimicrobial-susceptibility profiles.8,11,13,22,30,31,36 Traditional methods of SA-SCV detection and identification are based on unusual morphological appearances and biochemical reactions observed in SA-SCV isolates.5,11 Clinical SA-SCV–specimen isolates have general morphological characteristics of slow growth and 10 times smaller, nonpigmented, and nonhemolytic colonies on mannitol salt agar, tryptic soy agar, sheep blood agar, rose bengal agar, clindamycin blood agar, and chocolate agar (CHOC).5,8,11,22 CHROMID S. aureus Elite agar (SAIDE) showed more rapid and reliable species identification for SA than CHOC, in which 92.5% of SA was identified as green growth on SAIDE without dependence on auxotrophisms to hemin, menadione, or thymidine.8 Additionally, SA-SCV isolates generally exhibit reduced coagulase production and increased aminoglycoside and cell-wall–active antibiotic resistance in AST.5,8,11 Additional species identification methods of ID color catalase test (Slidex Staph Plus, bioMérieux) can further confirm isolation of SA species rather than commensal organisms.11,22 SA-SCVs also have a recognizable fingerprint observed on Fourier-transform infrared spectroscopy that can lead to further detection and confirmation of SA-SCVs.11
Aside from traditional diagnostic procedures, the implementation of matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry (MS) may render successful identification of SA-SCVs by comparing the spectra of the bioanalytes in an SA-SCV isolate with the established SA-SCV MS profile in the identification software.37,56,57 Previous studies describe the use of MALDI-TOF MS to identify SA and Enterococcus faecium SCVs.36,57,58 The use of high-performance liquid-chromatography MS can also provide a clear distinction between WT-SA and SA-SCVs based on changes in their metabolic profiles during the phenotypic switch and the unique metabolic SA-SCV signature.59 Moreover, proteomic analysis of WT-SA and SA-SCVs results in distinct protein profiles, such as SA-SCVs possessing larger amounts of induced proteins that are involved in glycolytic and fermentation pathways compared with their parent cells.60,61
SA-specific polymerase-chain-reaction (PCR) assays can be used to verify that observed SA-SCV isolates are indeed affiliated with the SA species.11,37 Multiple gene targets have proven useful for SA identification via PCR, including the genes nuc, coa, femA, eap, and sodM, which are not associated with mutation in SA-SCV and should be conserved between WT-SA and SA-SCVs.22,43,62⇓-64 PCR for aforementioned genes may provide additional discrimination of WT-SA from coagulase-negative Staphylococci, the latter being characteristic of SA-SCVs.65 The sensitive and specific identification of SA can permit reliable diagnosis of the species association of SCV isolates.37,64
A diverse range of detection methods may be used to determine the presence of SA-SCVs as a component of chronic, recurrent, and persistent infections. However, the growing concern of such infections echoes the current lack of a standardized diagnostic criterion in the detection and identification of SA-SCVs from a clinical standpoint. Therefore, the clinical laboratory should actively examine specimens for SA-SCVs and include detection methods in routine diagnostic procedures, lest they remain undetected or misdiagnosed, leading to further infection and potential unresponsiveness to antimicrobial therapy.5,11,36,42 Further studies should be conducted to determine which diagnostic methodology would be optimal to identify SA-SCVs from patient specimens.
IMPACT ON ANTIMICROBIAL SUSCEPTIBILITY
SA-SCVs experience reduced susceptibility to antimicrobials and antiseptics.5,10,11,14,24,29,35 Altered AST results from reduced bacterial-energy generation, intracellular transport, and downregulation of cell-wall synthesis and toxin production caused by environmental stress.11,14,29,32 Drug-resistance profiles show that SA-SCVs have increased resistance to aminoglycosides, sulfa drugs, cationic peptides, and cell-wall–active antibiotics.8,10,14,22,24,28,36 Aminoglycoside MICs were found 8- to 32-times higher for SA-SCVs when compared with MICs of the WT-SA phenotype; gentamicin MIC was notably 32-times higher for the SA-SCV phenotype.5,11,29,30,47
Gene mutations involved in the biosynthesis of thymidine, thiamin, menadione, and hemin lead to reduced functions of metabolic pathways, specifically ETC or tricarboxylic-acid cycle.6,14,25,26,28 Decreased electron transport impairs antimicrobial compound uptake and aminoglycoside and antifolate activities.11,13,14,24 Interruption of ETC is advantageous for SA-SCVs and antimicrobial resistance, such as intracellular persistence in nonprofessional phagocytes and increased biofilm formation.8,22 Mutations that inhibit respiration and metabolism reduce ATP production that is required in WT-SA for rapid growth.14 SA-SCVs exhibit longer generation times and slower growth in comparison with WT-SA because cell-wall synthesis requires large quantities of ATP.5,6,11,25 Slowed growth and metabolism then decrease cross-membrane potential and uptake of cationic-antimicrobial compounds, ultimately reducing aminoglycoside susceptibility.14,24 Aminoglycoside resistance is a direct result of ETC inhibition because uptake is dependent on membrane potential created by electron transport.11,14 Therefore, SA-SCV persistence and resistance to aminoglycosides is a major trade-off for reduction in growth rate.14
SA-SCVs also exhibit increased biofilm formation and reduced virulence to optimize their persistence within infection.9,28,39,41 In polymicrobial infections, PA was shown to protect SA with antistaphylococcal compounds in the presence of vancomycin and aminoglycosides, such as tobramycin and streptomycin.30,32,34,39,41 PA produces HQNO, pyocyanin, and siderophores that interfere with SA ETC, causing them to switch from respiration to grow fermentatively.5,32,34,41 Anoxic—or oxygen-limited—conditions and fermentative lifestyle inhibit WT-SA growth without killing it, slowing growth and selecting for SA-SCVs.6,14,30,32 Ultimately, PA induction of SA-SCV phenotype promotes viability and biofilm production in coculture with SA, contributing to protection and decreased aminoglycoside sensitivity.30,34,41 Different doubling times among SA-SCVs, WT-SA, commensal organisms, and other wound pathogens, even in small concentrations, cause the SA-SCVs to become rapidly replaced in liquid culture.11,14,36 Therefore, AST becomes a major challenge because SA-SCVs are easily overgrown and unconsidered, leaving them undetected.5,11,14,29,30
SA-SCVs can enter and persist within host cells, which contributes to reduced antimicrobial susceptibility.5,8,15,22,26 SA-SCVs efficiently invade nonphagocytic cells because of high expression of adhesion-fibronectin–binding protein and downregulation of α-toxin and proteinase.9,11,22,26,28,36,39 This is a mechanism to evade immune-system mechanisms, such as antibodies and complement.9,26,36 Ultimately, residence within host cells for long periods of time confers protection against antimicrobials as well; however, continued antimicrobial use causes mutations that select for the SA-SCV phenotype and increases their occurrence, persistence, and genetic variation.9,24,26,36 Therefore, intracellular persistence and slow growth ultimately lead to prolonged antimicrobial therapy, causing altered drug-resistance profiles and increased resistance to aminoglycosides. Importantly, SA-SCV phenotype is unstable and can revert to a highly virulent and rapidly growing form under the right conditions.9,22,25 SA-SCVs can therefore combine the ability to survive persistently with the propensity to generate fast-growing offspring when the right conditions are met to re-establish major infection.14
TREATMENT GUIDELINES AND RECOMMENDATIONS
SA-SCVs are a burdensome, fastidious organism to actively consider in clinical diagnosis and treatment, and this phenotype should be considered distinct from WT-SA. Treatment recommendations for SA-SCV infections vary based on the infection presented, further highlighting the importance of accurate diagnostic procedures.22 In cases of prosthesis or medical device infections, SA-SCVs readily attach to the devices postcontamination and are more resistant upon adherence.5,22 SA-SCVs respond poorly to prolonged antimicrobial therapy.11 Therefore, the current recommended treatment requires removal of all infected prosthesis, tissue, and foreign material followed by antibiotics prior to reintroduction of the prosthetic device.22
When treating SA-SCV infections with antimicrobial therapy, it is recommended to avoid aminoglycosides because of the phenotype’s altered antimicrobial-susceptibility profiles. Treatment with aminoglycosides can favor emergence of SA-SCVs and increase persistence.39 It has also been found that treatment with TMP-SMX induces the formation of SA-SCVs in diabetic foot ulcers and patients with CF.15 Patients treated with antifolates or antibiotics for over 1 month exhibit SA-SCV selection; therefore, prior long-term antimicrobial treatment also favors induction and increased persistence.13,15 SA-SCVs require immediate combination antibiotic therapy, which has been shown to successfully treat SA-SCV infections via rifampin with a fluoroquinolone rather than prolonged antimicrobial treatment.5,11 SA-SCVs have also shown response to flucloxacillin and rifampin combination therapy.35
Decreased SA-SCV antimicrobial susceptibility causes chronic and recurrent infection because of poor response to limited treatment options, which highlights the importance of accurate detection.31,53 To provide an accurate diagnosis of SA-SCVs, clinicians must consider the sum of clinical signs and symptoms, blood test results, and radiography results in addition to the microbiological workup.5 The slow growth and reduced metabolism of SA-SCVs cause them to be detected only after 48–72 hours of culture; therefore, prolonging the culture time is recommended to increase detection likelihood.5,28 Also, because of SA-SCV and PA coinfection frequency, it is recommended that when PA is detected in chronic or recurrent infections, SA-SCVs should be actively investigated.42
There is currently no universally appropriate protocol to treat SA-SCV infections. The incidence and prevalence of chronic infections resonates the absence of and the need for a standardized diagnostic methodology for SA-SCVs in a clinical setting. Further investigations should be conducted to determine a clinically appropriate standard within the clinical laboratory to optimally identify and treat SA-SCVs because this will help necessitate the translation of SA-SCV research from the benchtop to the clinical impact of reduction in chronic and persistent infections.
CONCLUSIONS
SA-SCVs are a metabolically deficient phenotype of the common opportunistic pathogen SA that are widely associated with recurrent and persistent infections. This phenotype is difficult to treat because of decreased antimicrobial susceptibility, intracellular persistence, and increased biofilm formation. The phenotype is metabolically dormant because of auxotrophism of hemin, menadione, or thymidine required for ETC-component biosynthesis. The SA-SCV phenotype is induced by environmental stressors, such as anoxic conditions, polymicrobial infection with PA, and pressure from certain classes of antibiotics. SA-SCVs exhibit unusual phenotypic and biochemical characteristics that distinguish it from WT-SA and can lead to misidentification or nonidentification in routine diagnostic procedures. Because of misdiagnosis, serious clinical implications arise from SA-SCV infections. This phenotype has been described in clinical cases of chronic infections, such as CF, SSTIs, osteomyelitis, foreign-body–associated infections, device-related infections, and others. Once induced by environmental stressors, SA-SCVs will often persist until the stressors are diminished, allowing them to revert to WT-SA and reinfect the host indefinitely until accurately detected and treated. Currently, there is not sufficient research on the mechanisms of pathogenesis or proposed treatment methods for these chronic infections. This highlights the importance of developing novel routine diagnostic methods to identify SA-SCVs in recurrent and persistent infections. Through active examination of the role of SA-SCV in infectious processes, elucidating new detection methods, and exploring novel treatment methods, the clinical implications of patient mortality and morbidity rates resulting from SA-SCV infection could diminish.
ACKNOWLEDGMENTS
This review article culminated from the background research conducted for the funded 2018–2019 American Society for Clinical Laboratory Science member research grant (proposal number 18-0761). We would also like to thank Dr Catherine Wakeman, Texas Tech University Department of Biological Sciences, for providing bacterial strains imaged in Figure 1.
- Received October 25, 2020.
- Accepted December 18, 2020.
American Society for Clinical Laboratory Science