This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Kathy Doig
, Michigan State University, doig{at}msu.edu
LEARNING OBJECTIVES
1. List test results in hematology, clinical chemistry, and urinalysis that may be abnormal in thrombotic thrombocytopenic purpura (TTP).
2. Discuss the etiology of the abnormal test results associated with TTP.
3. Recognize patterns of results consistent with TTP and fragmentation hemolysis.
4. Recognize spurious hematology parameters associated with in vivo red blood cell hemolysis and describe appropriate corrective actions.
ABSTRACT
Laboratory diagnosis of thrombotic thrombocytopenic purpura (TTP) often begins with routine laboratory tests; a complete blood count, clinical chemistry panel, and urinalysis. The classical findings may include anemia with schistocytes, thrombocytopenia, reticulocytosis or polychromasia, bilirubinemia, dark urine, and hemoglobinuria without red blood cells in the sediment. Additional findings, including decreased haptoglobin, can identify fragmentation as the cause for the hemolysis. The hemolysis in TTP arises from increased shear stress on red blood cells in arterioles and capillaries narrowed by microthrombi. Hemoglobinemia and schistocytes may generate spurious results in hematology analyzers that require correction before results can be released to the patient chart.
- CBC - complete blood count
- DIC - disseminated intravascular coagulation
- IPF - immature platelet fraction
- ITP - immune (idiopathic) thrombocytopenic purpura
- LDH - lactate dehydrogenase
- MAHA - microangiopathic hemolytic anemia
- MCH - mean cell hemoglobin
- MCHC - mean cell hemoglobin concentration
- MCV - mean cell volume
- MPV - mean platelet volume
- RDW - red cell distribution width
- TMA - thrombotic microangiopathy
- TTP - thrombotic thrombocytopenic purpura
INTRODUCTION
Thrombotic thrombocytopenic purpura (TTP) is one example of a microangiopathic hemolytic anemia (MAHA) wherein red blood cells are damaged in small blood vessels (chiefly arterioles and capillaries), resulting in hemolysis. When the condition causing the damage involves clots, the condition may also be called a thrombotic microangiopathy (TMA). The resulting hemolytic process and thrombocytopenia are detectable with routine hematology, clinical chemistry, and urinalysis testing.
HEMATOLOGIC FINDINGS IN TTP
The Complete Blood Count
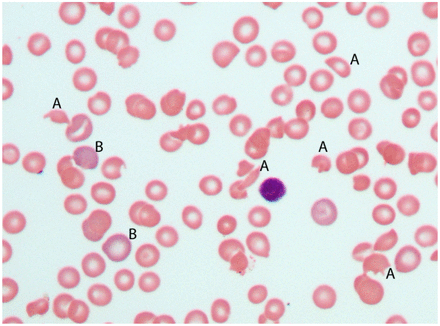
The complete blood count (CBC) may be the first clue to a care provider of a patient’s microangiopathic condition. Since TTP can be quickly fatal, the CBC is, therefore, a critical test. However, it is not diagnostically specific since other conditions present with a similar blood picture of hemolysis and thrombocytopenia. Such conditions include Shiga toxin–mediated hemolytic uremic syndrome, complement-mediated TMA, and drug-mediated TMA as well as other conditions.1 The CBC will typically demonstrate anemia with schistocytes and an elevated red cell distribution width (RDW), thrombocytopenia with elevated mean platelet volume (MPV) and immature platelet fraction (IPF), normal white blood cell parameters, and perhaps polychromasia. The laboratory results of the patient case provided in Table 1 demonstrate this classical presentation. Figure 1 shows the typical morphological presentation.
Admission laboratory values for a 40-year-old woman who was seen in the emergency department with abdominal pain over 3 days
Typical blood film in thrombotic thrombocytopenic purpura (TTP). This peripheral blood film from a patient with TTP demonstrates schistocytes (A), polychromasia (B), and thrombocytopenia, since no platelets are seen. Anisocytosis is reflected in the red blood cell size variation from (A) to (B) (magnification 500×).
Although MAHA with thrombocytopenia is a classic presentation in TTP, patients may have the condition without these signs. This is particularly true in chronic TTP, wherein a compensatory increase in platelet production can mask thrombocytopenia, and reticulocytosis can minimize a mild anemia.2 These compensatory responses can impact CBC findings. For example, the schistocytes will likely lower the mean cell volume (MCV) from the patient’s usual value but not necessarily below the reference interval. A reticulocytosis can then increase the MCV above what is usual for the patient but not necessarily outside the reference interval. These 2 factors may counteract each other, resulting in an MCV within the reference range, as is the case for the patient results in Table 1. However, the RDW, a measure of anisocytosis, is still expected to increase. If sufficiently numerous, schistocytes alone should increase the RDW. However, the presence of reticulocytes as well may increase it further since the variation of cell sizes between schistocytes and reticulocytes would be even larger (Figure 1).
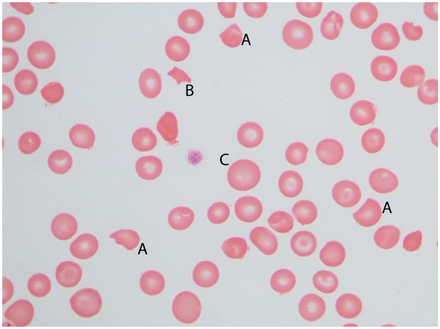
Compensatory platelet production to replace platelets consumed by the microthrombi would tend to increase the MPV. As platelets age during their time in the circulation, they become smaller. With increased thrombocytopoiesis, more platelets that are younger and, hence, larger enter the circulation from the marrow. The proportion of larger platelets increases and may raise the MPV. Once again, the rise may not lead to an MPV outside the reference interval. Figure 2 demonstrates a platelet of slightly larger size.
Platelets and shistocytes in thrombotic thrombocytopenic purpura. This field demonstrates thrombocytopenia with only a single large platelet in the center of the field. Shistocytes are also seen, including several keratocytes (A) and a schizocyte (B). Anisocytosis is notable in the size variation between (B) and (C) (magnification 1000×).
Studies have aimed to set a cut-off value to identify MPV levels associated with peripheral destruction of platelets and compensatory thrombocytopoiesis. The goal is to distinguish thrombocytopenia due to peripheral destruction, as in TTP, from hypoproduction associated with marrow disease. Most such studies enroll far more patients with immune-mediated peripheral platelet destruction, such as immune (idiopathic) thrombocytopenic purpura (ITP), than TTP patients. This is because ITP it is far more common than TTP. Furthermore, the range of normal platelet sizes may vary from instrument to instrument and from one study population to another so that decreases and elevations are difficult to compare across studies.3,4 Thus, no specific value that can point to peripheral destruction has been established to date. As is often the case, a comparison to the patient’s own values prior to the onset of thrombocytopenia is probably the best comparison for judging a possible MPV rise.
Although platelet MPV is higher for younger platelets, the parameter that more specifically assesses the age of platelets is the IPF. Flow cytometric analysis of platelets detects RNA content. Since RNA content declines as platelets age, younger platelets can be identified by their higher RNA content and enumerated separately.5 Thus, a rise in the IPF is expected to parallel a rise in MPV in conditions with peripheral consumption of platelets. The results in Table 1 reflect this.
Mechanism of Hemolysis in TMAs
Anemia is a secondary effect of microangiopathic thrombotic conditions. If the thrombosis can be stopped, the anemia will correct. A hallmark finding of the anemia in microangiopathies like TTP is the shistocytes: red blood cells fragmented in the vasculature leaving irregularly shaped red blood cell shards that are readily seen on a stained blood film (Figure 1). Fragmentation can occur whenever the flexible membrane of the red blood cell is breached by impacts that exceed its ability to deform and return to normal. As such, 2 factors, the cell’s flexibility and exposure to impacts, will contribute to hemolysis.
The flexibility of red blood cells is affected by the surface-area-to-volume ratio and the viscosity of the red cell cytoplasm.6 Changes in cell shape affect surface-area-to-volume ratio. It is easy to imagine that a spherocyte with the lowest surface-area-to-volume ratio (ie, highest volume for least surface area) is very rigid and unable to flex when entering a capillary. Target cells demonstrate the opposite effect, having disproportionately more membrane (surface area) for their volume. Thus, they are very flexible. In fact, the target appearance is an artefactual bulge of excess membrane in the center of the red cell pallor area of cells when flattened on a slide.7 For most patients with TTP, the effect of red cell surface area to volume is not an initial contributor to their hemolysis since their cells are normal. However, once formed, fragmented cells are innately more rigid and fragile.8
A second factor affecting cell flexibility is viscosity inside the cell itself. The normal shape integrity of red cells depends largely on the cytoplasmic viscosity of red blood cells when contained by the membrane of lipids and proteins. The concentration of the internal hemoglobin solution is the major contributor to the internal viscosity of red cells; thus, hypochromic cells are more flexible since they are less viscous internally. As long as the viscosity inside the cells is higher than the surrounding medium, the red cells will resist deforming since the interior is effectively pushing outward against the membrane.6 Thus, it acts like a water-filled balloon having some flexibility but that is, of course, still being limited in its flexibility. When the viscosity of the surrounding medium is greater than the viscosity inside the cells, it exerts pressure from the outside onto the cell, promoting shape distortion and possible rupture.6,9 Again, typically, this is not a factor for TTP patients, as their cells and plasma are normal. Therefore, the hemolysis in TTP mostly involves the impacts experienced by normal cells in normal plasma. One such impact is the endothelial shear force in blood vessels.
Red blood cells can rupture when the endothelial shear force within the vessels exceeds the cells’ ability to deform and regain normal shape without rupture. Shear force is the force exerted on a body (in this case the red cell) that pushes one part of that body in one direction and another part of the body in another direction, creating shear, that is, shifting of the parts relative to one another in parallel planes. A common analogy is a deck of cards on a table, in which the deck is pressed and pushed in one direction while the table acts in the other direction to prevent it from moving. As a result, the cards are displaced from one another in parallel. Another analogy is what happens when you run your hand up your cheek. Each of these demonstrates that parallel planes remain parallel, but they are shifted spatially in relationship to each other.
To apply this concept to red blood cells, imagine a cell interacting with the epithelial surface of the vessel, which slows it down on the side facing the vessel wall. However, if the blood is flowing swiftly along the other side of the cell, it will push that side forward. This can cause red blood cells just to tumble along the vessel wall without lysis.10 However, fast-flowing blood could cause the cell to rupture when one side is pushed forward forcefully while the other remains relatively anchored to the vessel wall.
Shear force in blood vessels depends on 3 factors.2,9 The first is the flow rate; that is, how fast the blood is moving. Faster-moving blood, as in arteries, experiences more shear force relative to the stationary vessel wall. The second factor is the size of the vessel. Shear stress is higher in smaller vessels wherein the cells are more likely to interact with the vessel wall. In addition, the third factor is the viscosity of the medium in which the red cells are suspended, that is, the plasma. One can imagine that if the viscosity of the plasma increases, it places greater shear stress on the red blood cells moving through it.9 It would be like the difference between moving your hand up your cheek wet with water or a cheek wet with honey. In addition, as discussed previously, a higher-viscosity medium promotes shape distortion.
This background on factors affecting shear force and red cell integrity can explain why red cells rupture when thrombi narrow arterioles. The plasma viscosity, red cell cytoplasmic viscosity, and red cell surface-area-to-volume ratios are normal for most patients with TMA. However, even in healthy individuals, arterioles are sites of red cell trauma due to shear stress. As blood, moving from arteries at relatively high flow rate, is channeled into smaller vessels, shear force increases. Approximately 10% of the normal daily loss of red blood cells occurs at such sites, probably mostly affecting older cells with reduced energy production and, thus, reduced ability to flex and regain shape.11 The additional narrowing, or stenosis, of arterioles in thrombotic microangiopathic conditions exacerbates the shear force and results in higher-than-normal levels of hemolysis. In contrast, hemolysis does not occur in conditions with venous thrombi because the blood flow is slower and veins get progressively larger.2 Thus, it is the combination of relatively high flow rate and excessively narrowed arterioles that increases shear stress and results in the hemolysis in TTP and other TMA conditions with arteriolar thrombi.
A second mechanism of hemolysis that may be pertinent in TTP, though a minor component, is slicing of red blood cells by fibrin strands across capillaries. This process is likened to a wire cheese slicer slicing through a block of cheese. Although this mechanism is prominent in other microangiopathic hemolytic processes like disseminated intravascular coagulation (DIC), it is not a significant factor in TTP owing to little fibrin contribution to the thrombi.2
There is another factor contributing to hemolysis but not schistocyte formation. Red blood cells that become trapped in the microthrombi will lyse. Yet, because they are trapped, they do not contribute to circulating schistocytes. In TTP, this is a relatively modest contribution to the overall hemolytic process since the thrombi are composed of relatively little fibrin to trap cells as compared with other conditions like DIC.2
MORPHOLOGY OF SCHISTOCYTES
Mechanical fragmentation of red blood cells leads to remnants of varying sizes and irregular shapes. Bessis took care to distinguish cells that would appear to be more than 50% of a cell’s volume from smaller fragments.8 He referred to the larger fragment as a keratocyte from the Greek “kerato” meaning horn. This was because the cells may have 2 spikes like animal horns, though he said they could have up to 6. Such cells are visible in Figure 2. Others have called these “bite” cells because they appear like a cookie with a bite out of it. This more common term is perhaps more widely used than Bessis’ proposed Greek-based nomenclature. However, bite-shaped cells should not be construed to form due to the action of macrophages taking a bite out of the cells. Macrophage actions on red cells typically lead to spherocytes rather than fragments of any size.
If a keratocyte is the larger portion of a fragmented red blood cell, then the smaller fragment is, using Bessis’ terminology, a schizocyte.12 The Greek root, “schizo,” means split. Although Bessis made the distinction between schizocytes and keratocytes, he acknowledged the common mechanism of their formation.12 Therefore, they are expected to be seen in the same conditions, and both can be expected for a given patient. It is common for them to be lumped together under the term schistocytes, which comes from a second Greek root for “split.” The patient results in Table 1 reported schistocytes on day 1, whereas bite cells were reported on day 4. Of course, consistent use of reporting terminology is important to appropriate interpretation by care providers.
SPURIOUS HEMATOLOGY RESULTS FROM FRAGMENTING OF RED BLOOD CELLS
Hemolysis that occurs in the sample collection process and sample tube can be corrected by collecting a new sample. However, with in vivo hemolysis, as in TTP, hemoglobinemia will be present in a recollected sample as well, creating the possibility for spurious hematology parameters.
The hemoglobin value reported in a CBC is intended to reflect the hemoglobin contained inside the red blood cells. When hemoglobin is present in the plasma, it is also measured by instruments that lyse cells and measure hemoglobin chemically. Thus, the reported hemoglobin value is falsely elevated. This value is then used in calculating red blood cell indices like the mean cell hemoglobin (MCH) and MCH concentration (MCHC) (or their trademarked equivalents), each of which will also be falsely elevated. Analyzers will typically flag these elevated values, particularly the MCHC, to draw operator attention to the possibility of spurious values. Protocols may vary by manufacturer for correcting the error. A common procedure used to correct for other plasma interferents, like lipemia, can be applied to hemoglobinemia also. The sample is centrifuged, and the plasma is removed and replaced with an equivalent volume of saline.13 The modified sample is retested, and, having removed the hemoglobin interference, the results can be reported. Hemoglobinemia must be substantial to create this problem, however.
Another possible spurious result can occur with electrical impedance counters when schistocytes are mistakenly counted as platelets because of their small size. In TTP, this false elevation of the platelet count could impact the assessment of thrombocytopenia, thus delaying proper diagnosis. Flags may alert the operator, though, since the platelet histogram is typically skewed by the schistocytes. A correct platelet count may be attainable using a flow cytometry counter that typically relies on the platelet RNA, thus preventing red cell interference. Though rarely performed anymore, a hemacytometer count will isolate the platelets for counting since the diluent lyses the red cells.
It is also possible that when small red cell fragments are counted as platelets, the red blood cell count will be falsely lower and the MCV falsely higher since they are not included.13 The magnitude of the impact is negligible, however, since red blood cell counts are 1000 times higher than platelet counts. As an example, 100 000/µL schistocytes added to a 75 000/µL platelet count is a clinically significant difference, whereas 100 000/µL schistocytes missed in a 5 000 000/µL red blood cell count is not a meaningful difference. The resulting MCV is also not meaningfully affected. Thus, no technical correction for the uncounted red blood cells (schistocytes) is needed.
CLINICAL CHEMISTRY FINDINGS IN TTP
Intravascular red blood cell lysis releases hemoglobin into the plasma immediately. Hemoglobinemia is transient with each hemolytic event, though it can be ongoing if the hemolytic process is chronic. The free hemoglobin circulates in alpha-beta dimers that bind to circulating haptoglobin to prevent loss of the associated iron in the urine.14 The hemoglobin dimer-haptoglobin complex binds to CD163 on the surface of macrophages throughout the body but mainly in the spleen, liver, and bone marrow, thus removing it from the plasma.15 Haptoglobin is not recycled to the plasma by the macrophages, nor is there a compensatory increase in production, so levels drop within the first few days subsequent to a hemolytic event. Once hemolysis subsides and the hemoglobin is cleared from the plasma via this mechanism, haptoglobin levels return to normal. The haptoglobin level for the patient in Table 1 demonstrates this dramatic drop on day 1. On day 4 of effective treatment, her haptoglobin level had risen to 53 mg/dL. Thus, a reduced level of haptoglobin can be used to detect intravascular hemolysis and distinguish it from macrophage-mediated (extravascular) hemolysis. In the latter condition, the hemoglobin remains inside the red blood cell until it is ingested by a macrophage and lysis occurs inside a macrophage lysosome; thus, hemoglobinemia does not occur, and plasma haptoglobin is not consumed.
Macrophages catabolize the absorbed hemoglobin dimers. Globin chains are degraded and circulate in the amino acid pool. Heme oxygenase removes iron from heme-generating biliverdin and carbon monoxide. Biliverdin is then reduced to unconjugated bilirubin and is released into the bloodstream. Thus, there will be an increase in the serum total bilirubin and unconjugated bilirubin. This is not unique to fragmentation hemolysis, though, as these analytes also increase in macrophage-mediated hemolysis. The patient results in Table 1 demonstrate the elevation in total bilirubin.
Unconjugated bilirubin in the plasma binds to albumin and circulates to the liver where organic anion transporters actively move bilirubin into hepatocytes.16 The hepatocyte then conjugates the bilirubin to 2 glucuronide molecules, making the resulting conjugated, or direct, bilirubin water soluble. Conjugated bilirubin then enters the bile and is ultimately converted to urobilinogen in the intestine. Plasma direct bilirubin typically remains within reference intervals during hemolytic processes.
Red cell lysis can release hemoglobin molecules at a concentration 1000 times greater than local plasma haptoglobin concentration.17 Thus, the local haptoglobin is quickly saturated. Remaining free hemoglobin dimers in the plasma autoxidize rapidly and release free heme.18 A second iron salvage molecule, hemopexin is able to bind the oxidized heme, called metheme or hemin. Hemopexin-metheme complexes then bind to CD 91 on hepatocytes and are internalized, and the heme is catabolized as described above. Hemopexin synthesis in hepatocytes increases as intracellular concentrations of heme increase.19 Unlike haptoglobin, there is no reduction in hemopexin in the plasma relative to a hemolytic event. Thus, measurement of hemopexin is rarely helpful in diagnosis of hemolysis.
Plasma lactate dehydrogenase (LDH) released from fragmented red blood cells will rise to well above the reference range (see Table 1) owing to the dramatic increase in the red cell fraction.20 LDH concentration or isoenzyme ratios are an important monitoring tool in treatment of intravascular hemolysis.20 Serum potassium and folate levels will also rise above the reference range after a hemolytic episode due to release from red blood cell cytoplasm.21
Creatinine may be elevated, especially in cases of hereditary TTP; however, in acquired TTP, frank renal failure is less typical, so values may be only modestly elevated, if at all, as is the case with the patient results in Table 1.2,22
URINALYSIS FINDINGS IN TTP
In severe hemolytic events, the available plasma haptoglobin will become saturated and removed from the plasma. Any remaining free (met)hemoglobin enters the glomerulus of the kidney and is filtered into the urine. There is some nonspecific protein absorption in the proximal tubule cells by the megalin-cubilin-amnionless system.23 However, this system is quickly overwhelmed, leaving methemoglobin in the filtrate, which may contribute to the slightly increased protein value in the results in Table 1. Since methemoglobin is a brown pigment, the color of the urine is dark. With severe hemolysis, the urine may be root-beer colored. The dark yellow of the urine in the results in Table 1 likely results from methemoglobin.
Urinary biochemical test strips will be positive for blood because of hemoglobin, whereas the microscopic examination will be negative for red blood cells, thus pointing to a hemolytic process. Bilirubin is negative because direct serum bilirubin is not elevated during hemolysis. The results in Table 1 demonstrate this pattern of results.
Urobilinogen is a water-soluble compound that readily enters the portal circulation from the intestines and is circulated for excretion through the liver again. A small fraction of the urobilinogen that is not excreted by the liver remains in the plasma and will be excreted via the urine since it filters through the glomerulus and is water soluble. For this reason, small amounts of urobilinogen are present in normal urine. However, in hemolytic processes when there is more than the usual amount of bilirubin converted to increased amounts of urobilinogen in the intestine, urobilinogen can be increased in the urine as in the results in Table 1.
Proximal tubular cells contain heme-oxygenase–like macrophages and hepatocytes, so they are able to remove iron from heme and form bilirubin.24 Any stored iron that converts to hemosiderin will cause sloughed tubule cells to stain positive with Prussian Blue.25
SUMMARY
CBCs, comprehensive metabolic panels, and urinalyses do not provide results that are pathognomonic or diagnostic for TTP. Rather, the pattern of results across these test panels can point to a fragmentation hemolytic process with thrombocytopenia. That is sufficient to consider TTP. Laboratorians should recognize the constellation of findings and alert appropriate care providers since TTP can be rapidly fatal.
ACKNOWLEDGMENTS
The authors express appreciation to Kimberley Kabb, Scientific Communication and Education Manager, Sysmex, Inc.
Original photography by Susan McQuiston, JD, MT(ASCP)
- Received February 12, 2020.
- Accepted June 4, 2020.
American Society for Clinical Laboratory Science