This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Vishal Chhetri
- Nima Nima
- Ugyen Dophu
- Kinga Jamphel
- Rinxin Jamtsho
- Kuenzang Dorji
- Sacha Tenzin
- Jigme Tenzin
- Choney Wangmo
- Gelephu Central Regional Referral Hospital
- Pathology and Laboratory Medicine Unit, Wangdue Hospital, DMS, MoH, Bhutan
- Ministry of Health, Thimphu, Bhutan
- Drug Regulatory Authority, Thimphu, Bhutan
- Ministry of Health, Thimphu, Bhutan
- Ministry of Health, Thimphu, Bhutan
- Eastern Regional Referral Hospital, Mongar, MoH, Bhutan
- Jigme Dorji Wangchuck Referral Hospital, Thimphu, Bhutan
- Pathology and Laboratory Medicine Unit, Wangdue Hospital, DMS, MoH, Bhutan
- Address for Correspondence: Vishal Chhetri
, Gelephu Central Regional Referral Hospital, vishal_srmc{at}yahoo.com
ABSTRACT
INTRODUCTION: The evidence-based practice in medicine has made the laboratory diagnostic imperative for optimal health care services. The quality approaches in laboratory medicine in Bhutan have been incorporated since the one last decade. The quality of the clinical chemistry in Bhutan has been compromised due to the erratic supply of the reagents or consumables, poor equipment maintenance, and lack of automachine. In order to address this predicament, the Reagent Rental System (RRS) was introduced in clinical chemistry in 2014. In this study, we evaluated the efficiency of RRS at clinical chemistry services in Bhutan.
METHODS: A set of questionnaires was developed and information was collected from 8 major hospitals with RRS. The information was also collected from the physicians and hospital administrators on RRS. Data entry was done using Microsoft Excel. Descriptive information is presented as frequencies, means, and percentages.
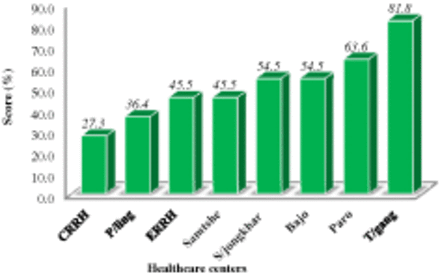
RESULTS: The overall satisfaction rate among the clinical chemistry laboratories using RRS was found to be 51.1%. Among the participating laboratories, Tashigang General Hospital Laboratory showed the highest satisfaction rate (81.8%). The Central Regional Referral Hospital Laboratory was found to be the least satisfied (27.2%). The implementation of RRS has minimized the coefficient of variation (CV) from 27.3% in 2011 to 5.1% in 2017. In the last 4 years (2014–2017), the average service interruption was reduced to 40.5 days from 14 months in the past. The participating laboratories were found to be satisfied with the analytical performance of the auto-analyzer; however, the nontechnical factors have negatively influenced the efficiency of RRS to improve the quality of the service. The physicians and hospital administrators also found that RRS has improved the quality of the service with reliable patient reports.
CONCLUSIONS: The RRS has improved the quality of the clinical chemistry services by improving the supply of the reagents and consumables, equipment maintenance, and CV.
- ASO - anti–streptolysin O
- CV - coefficient of variation
- IQC - internal quality control
- JDWNRH - Jigme Dorji Wangchuck National Referral Hospital
- QA - quality assurance
- RF - rheumatoid factor
- RRC - Reagent Rental Contract
- RRS - Reagent Rental System
- TAT - turnaround time
- UPS - uninterrupted power supply
INTRODUCTION
The paradigm shifting into evidence-based medicine has made the laboratory diagnosis an integral part of a health care system. Although there is a minimal consensus, approximately 70% of the medical decisions regarding the diagnosis and treatments are reported to be based on the clinical laboratory findings.1,2 The quality of laboratory medicine service is imperative to provide optimal health services. The quality assurance (QA) in laboratory medicine includes a wide range of activities specifically designed to improve the reliability of the test results.3 Most of the QA emphasis in laboratory medicine has been placed in improving the precision and accuracy of the test results.
Since the beginning of the laboratory medicine services in Bhutan in 1975, the clinical chemistry services were frequently interrupted because of the erratic supply of the reagents and consumables, poor equipment maintenance, and frequent breakdown. This quality predicament is primarily due to usage of multibrands of reagents. In order to elucidate this chronic quality crisis, the Reagent Rental System (RRS) was introduced in clinical chemistry at Jigme Dorji Wangchuck National Referral Hospital (JDWNRH) in 2013. The technical committee, after considering the technical features of the analyzer and cost/benefit analysis, installed a fully automated chemistry analyzer CS-600 & CS-300 (Diuri) based on the principle of photometry and light transmittance at various wavelengths at JDWNRH. The initial observation indicated an improvement in the quality of the service through improvement of the supply of reagents and consumables, functionality of the analyzer, and reducing turnaround time (TAT). Considering the positive impression of RRS, it was then stretched to 2 regional referral hospital and 6 district hospitals. The automated clinical chemistry analyzer EM-360/EM-200 (Erba Mannheim) was selected and installed in 8 health care centers in 2014. In this study, we evaluated the efficiency of the RRS to improve the clinical chemistry service in Bhutan with regard to the analytical requirements and service delivery.
METHODS AND MATERIALS
The RRS technical committee developed and verified a set of questionnaires consisting of 2 parts; the first part consists of 11 questions focusing on the end-user satisfaction and the second part focusing on the ways to improve the next RRS scheme. The questionnaires were sent to all 8 laboratories and collected their feedback on the RRS. The physicians and administrators were also asked to provide their feedback on RRS to enhance laboratory medicine services.
Data entry was done using Epi-info 7.0 and Microsoft Excel. Descriptive information is presented as frequencies, means, and percentages; p ≤ 0.05 is considered statistically significant.
RESULTS
The overall satisfaction rate among the clinical chemistry laboratory using RRS is 51.1%. Among the participating clinical chemistry laboratories, Tashigang General Hospital Laboratory showed the highest satisfaction rate (81.8%), and the Central Regional Referral Hospital was found to be the least satisfied (27.3%) (Figure 1).
The satisfaction rate among 8 clinical chemistry laboratories running on Reagent Rental System.
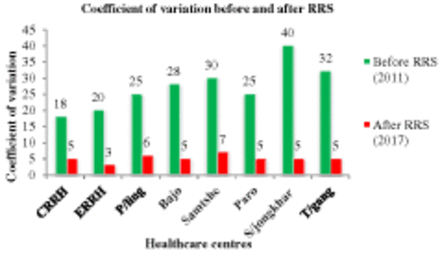
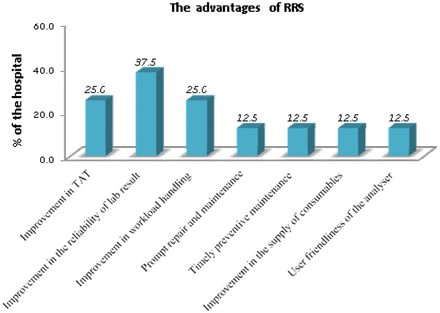
The introduction of RRS has decreased the average coefficient of variation (CV) of internal quality control (IQC) from 27.3% in 2011 to 5.1% in 2017 (Figure 2). The average service interruption in the first 4 years after the introduction of RRS was 40.5 days, as compared with 14 months in the past. All the participating laboratories were found to be satisfied with the analytical performance (precision and accuracy) of the equipment. The current equipment is reported to be robust by 75.0% (n = 6) of the laboratories. The end-user satisfaction rates for the supply of the reagents, consumables, and accessories; maintenance service offered by the biomedical engineer; engineer’s response time to an equipment breakdown; training or information during the equipment maintenance; and vendors’ services are 37.5% (n = 3), 37.5% (n = 3), 37.5% (n = 3), 62.5% (n = 5), and 50.0% (n = 4), respectively. The most common end-user recommendation for the improvement of RRS was the requirement of a sustained supply of adequate quality control materials and calibrators (62.5%, n = 5). The issuance of uninterrupted power supply (UPS) battery to ensure constant power supply (75%, n = 6) was recommended. Among 23 different types of troubleshooting errors were faced using EM-360 and EM-200 analyzer; the most frequent were VOD error (37.5%, n = 3), frequent quality control failure in some parameters (37.5%, n = 3), and analyzer not responding at COM (37.5%, n = 3). The anti–streptolysin O (ASO) and rheumatoid factor (RF) analyses were found to be recurrent QC deranged parameters by 87.5% (n = 7). The RRS improved the reliability of the test results (37.5%), reduced the TAT (25.5%) and improved throughput of the analyzer (efficiency of laboratory) (25.5%) (Figure 3).
The average coefficient of variation of clinical chemistry of individual health care centers before and after implementation of Reagent Rental System.
Impact of Reagent Rental System to improve the quality of laboratory medicine services.
DISCUSSION
The rapid advancement in laboratory technology and the range of available tests has frequently made laboratory medicine the lead in medical science. In most of the cases, a diagnostic or screening test for a disease condition is available for an effective medical intervention.4 This rapid progress has improved the evidence required for the medical practice; however, it has imposed a great challenge to clinical laboratories, particularly in developing countries, to keep up to date with the rapidly advancing technologies and generate quality-assured results. At present, there are 2 main ways to obtain an instrument in the medical laboratory: purchasing and renting.5 The conventional purchase system requires a huge initial capital investment, and, furthermore, the equipment gets obsolete rapidly. In contrast, the renting system does not require a capital investment and additionally equips the laboratory with the cutting-edge technology. This advantage has made a renting system a globally preferred means to acquire laboratory equipment.5,6 In RRS, the equipment cost, service charges, and other relevant expenses are included in the reagent price; therefore, the decision to rent or purchase clinical laboratory equipment should be based on the cost/benefit analysis and other relevant factors.7
Laboratory equipment in Bhutan is conventionally purchased through a proper procurement system. However, constant financial constraints, rapidly advancing laboratory technology and high equipment maintenance cost have made it unsuitable to own the equipment. Additionally, due to the limited number of vendors, the price of the reagents keeps escalating over the years, and the supply is erratic. To address the challenges, RRS was introduced in 2014 after comprehensive analysis. Majority of the Reagent Rental Contract (RRC) term in the United State is 60 months; however, the RRC term can vary from 12 to 84 months.7 The current clinical chemistry RRS is for the period of 60 months, and this term is recommendable for a future RRC since most of the laboratory equipment gets obsolete within 5 years.
The implementation of the RRS in clinical chemistry has satisfied all the participating laboratories; furthermore, physicians and hospital administrators also shared similar views. The RRS has improved the overall precision of the analysis, thereby increasing efficacy and reducing TAT.8 There is a wide variation in satisfaction rate among the participating laboratories, and this inconsistency may be primarily attributed to the number of equipment breakdowns in individual laboratories and accessibility to biomedical engineers.
All the participating laboratories were found to be satisfied with the analytical performance of the analyzer. The annual average IQC CV in 2011 before the introduction of RRS was 27.3%, which drastically improved to 5.1% in 2017 with the introduction of RRS. However, some nontechnical factors needed to be addressed. Among the nontechnical factors, the participating laboratories showed the least satisfaction regarding the biomedical engineers’ response time during an equipment breakdown, maintenance service, and supply of consumables. The suitable TAT of the biomedical engineer’s response to the equipment breakdown and the supply of the reagents and consumables needs to be clearly specified in future RRC. The prolonged TAT by the biomedical engineer can be solved by placing the biomedical engineer in country and training end users on basic troubleshooting. Among 16 recurrent QC deranged parameters, ASO and RF are the most frequent deranged parameters, followed by magnesium and C-reactive protein. Similarly, 23 different types of technical errors were faced while operating this analyzer. Although recurrent QC deranged test parameters and technical errors do not reflect the poor efficiency of the RRS, careful research about the technical functionally of the analyzer quoted in RRS can add up to the efficiency of the RRS to further improve the quality of the services.
The participating laboratories provided 17 recommendations for improvement in efficiency of the future RRS. Among the recommendations, providing a UPS and constant supply of the QC materials, reagents, and calibrators was recommended by all laboratories. The electrical supplies, particularly along the southern region, are inconsistent during monsoon season, and, furthermore, earthing in most of the health care centers is suboptimal. In this view, consistent electrical connection through suitable UPS backup and proper earthing can improve functionality of any laboratory equipment. Clinical laboratories in Bhutan have been frequently functioning without proper QC materials. The high cost of the QC materials, short shelf life, and poor transportation system has led to this quality crisis. For the betterment of the RRS, the price of the QC materials needs to be fixed for the entire RRS tenure and supplied in a timely manner. The calibration of the equipment is indispensable for the optimal reliability of any analyzer; therefore, the RRS term should specify the agent responsible for timely calibration.
The major limitation with the RRS is that the list of accessories of the equipment should be clearly mentioned in the contract, otherwise it will add cost to the end users. The other limitation is the RRS equipment must be brand-new equipment and not refurnished, as it will add to the running cost of equipment and need for frequent equipment calibration.
The current study collected open opinions from the physicians and hospital administrators; therefore, the future study can use a set of questionnaires to standardize the evaluation. Additionally, the future studies should also evaluate the patient’s satisfaction to better understand the overall efficiency of the RRS to improve the services. Moreover, future RRS in the country should present with the cost-per-test calculation and opt for partially open system, whereby there is the benefit of programming tests that are not available with the company owning the machine but are required for the patient care.
CONCLUSIONS
The decision to own or rent the clinical laboratory equipment should be primarily based on the cost/benefit analysis. The RRS is a suitable method to procure clinical laboratory equipment since it can evade the high capital investment and, moreover, equips the laboratory with the state-of-the-art technology. Furthermore, specific to our situation, the RRS can overcome the multibranding of reagents, rapidly increasing reagent and consumable price, scarcity of qualified biomedical engineers, and high equipment-maintenance cost. The RRS contract period of 60 months is recommended for the future RRS since most of the laboratory equipment becomes obsolete within 5 years. The implementation of RRS in the clinical chemistry laboratory has improved the quality of the services by improving the analytical precision, increasing efficacy, reducing TAT, and minimizing service interruptions. Efficiency of the RRS to improve the quality of the service is equally influenced by both technical and nontechnical factors; therefore, both factors should be equally considered to improve the future RRS. Finally, the current study indicates that the implementation of RRS in other clinical laboratory specialties can uplift the overall quality of laboratory medicine services.
ETHICAL APPROVAL
Ethical clearance was granted by Research Ethics Board of Health (REBH), Ministry of Health, Bhutan vide letter no: Ref. No. REBH/Approval/2019/092 dated 06/12/ 2019.
ACKNOWLEDGMENTS
We thank the respective hospital administrations for allowing the use of laboratory data. We also thank our colleagues in the hospital laboratories for assistance in different forms during data collection. We are also grateful to QASD and DoMSHI for the necessary support. No funding was received for this study in any form.
- Received September 10, 2020.
- Accepted December 13, 2020.
American Society for Clinical Laboratory Science