This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Weber State University
- Weber State University
- Weber State University
- Carter BloodCare
- Weber State University
- Weber State University
- Address for Correspondence: Jesus Rebolledo
, Weber State University, tigoragaa{at}yahoo.com
ABSTRACT
Polyagglutination (PA) is a rare condition that can occur during microbial infections where enzymes capable of modifying carbohydrates on the erythrocyte membrane are produced. These enzymes expose cryptantigens that react with naturally stimulated antibodies found in nearly all adult sera, except autologous sera. Certain lectins can agglutinate red blood cells (RBCs) carrying these cryptantigens. The agglutination patterns of test RBCs in lectin panels can provide presumptive evidence to identify major forms of PA. Lectin testing kit manufacturers within the United States have discontinued their production. However, immunohematology reference labs (IRLs) must continue offering PA testing under current Association for the Advancement of Blood & Biotherapies IRL Standards. Consequently, IRLs must adopt unapproved storage methods for expired lectin kits or create lectin reagents in-house. Quality control (QC) RBCs evaluating lectin reagent performance are often from patients with PA and are difficult to obtain. This study describes a method to produce a lectin reagent and QC RBCs in-house to efficiently and cost-effectively identify the T-activated form of PA. Additionally, we report the results of a national survey disseminated to evaluate the current status of lectin testing procedures in IRLs.
- AABB - Association for the Advancement of Blood & Biotherapies
- ABC - America’s Blood Centers
- EDTA - ethylenediaminetetraacetic acid
- g - gravities
- gm - grams
- IRB - Institutional Review Board
- IRL - immunohematology reference laboratory
- PA - polyagglutination
- PBS - phosphate-buffered saline
- QC - quality control
- RBC - red blood cell
- RPM - revolutions per minute
- RT - room temperature
INTRODUCTION
Although all forms of polyagglutination (PA) are relatively rare, the most common forms are due to exposure of the following cryptantigens via the action of bacterial enzymes: T, Tk, Th, and Tx.1⇓-3 Rarer forms of PA are due to inheritance of alleles that result in the formation of abnormal antigenic determinants and react with nearly all human sera.1 Persistent PA is associated with a somatic mutation leading to an enzyme deficiency and exposure of the cryptantigen Tn.2 Most adult sera contain naturally occurring antibodies that bind to cryptantigens and are produced after exposure to common environmental substances.4 The primary cryptantigen-binding antibody isotype is immunoglobulin M; therefore, the antibodies are capable of causing direct in vivo red blood cell (RBC) agglutination that can occlude blood vessels and lead to major complications throughout the body.5 Although PA is uncommon, it should always be considered when extra, unexpected hemagglutination results in ABO discrepancies and may also result in a positive direct antiglobulin test.6
Activation of the T antigen was first observed when RBC suspensions were left at room temperature (RT) for several hours and then agglutinated when mixed with ABO-compatible serum. It was hypothesized that enzyme-producing bacteria were responsible for this unexpected hemagglutination.7⇓-9 It is now known that several organisms, including pneumococci, streptococci, staphylococci, clostridia, Escherichia coli, Vibrio cholera, and the influenza virus, are capable of activating or exposing the T antigen and thus rendering the RBCs polyagglutinable.1
In vivo activation of the T antigen is usually a transient condition, which resolves within weeks to months once the infection has cleared.1 Although the serum from nearly all adults contains anti-T antibodies capable of agglutinating RBCs with activated T antigens, a lectin derived from the common peanut Arachis hypogaea is far more sensitive at detecting T-activated RBCs in vitro than the antibodies present in adult sera.10
The production of lectin kits that test for PA has been discontinued in the United States. However, immunohematology reference labs (IRLs) are required to offer PA testing under current Association for the Advancement of Blood & Biotherapies (AABB) IRL Standards.4 As a result, IRLs have adopted inconsistent storage methods for their PA testing materials or have begun producing their lectin reagents in-house. The in-house production of lectin reagents is not standardized within the United States, and quality control (QC) cells that express the cryptantigens are rare and difficult to procure. The procedures for lectin isolation described in Judd’s Methods in Immunohematology11 and in the AABB Technical Manual12 do not specify the precise shelf lives of lectin reagents, and the in vitro T-activation procedure described by Moulds13 does not specify the exact strain of S. pneumoniae nor the precise incubation time required to activate the T cryptantigen for the RBCs used to perform QC.
This study describes a detailed method to produce a long-lasting lectin reagent and QC RBCs in-house to test for the T-activated form of PA with supplies and equipment that are found within most IRLs. Furthermore, the results from a nationally disseminated survey are evaluated; the results identify the current status of lectin kit testing for PA within IRLs.
MATERIALS AND METHODS
Enzyme Collection
Neuraminidase is an enzyme produced by Streptococcus pneumoniae and is known to cleave carbohydrate branches of RBC surface antigens and expose the T antigen.11,13 S. pneumoniae (ATCC 49619) was streaked onto a sheep blood agar plate and incubated at 37 °C in a 3%–5% CO2 incubator for 24 hours. Optochin susceptibility and catalase tests were performed to confirm the identity of the species. A glucose solution was prepared by mixing 0.5 grams (gm) of dextrose with deionized water in a 100 mL volumetric flask. Four to 5 colonies were placed into 6 tubes, each with 25 mL of liquid containing 20 mL of trypticase soy broth and 5 mL of a 0.5% glucose solution. The broth was incubated for 24 hours to allow the S. pneumoniae to grow and produce neuraminidase. The broth was centrifuged at 450 × gravities (g) (1400 revolutions per minute [RPM]) for 5 minutes, and the supernatant was removed and filtered through a 0.45-micron filter.
Blood Preparation
In order to test the specificity and potency of the isolated lectin, QC cells were created by activating the T antigen in vitro following an adaptation of Moulds’s method.13 It was noted that the 1-hour incubation time specified in Moulds’s method was insufficient to activate the T antigen. Blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes through venipuncture. The blood was washed 3 times to remove the EDTA. The RBCs were confirmed to be O negative via the slide typing method. To activate the T antigen, a volume of 6 mL of the washed red cell suspension was mixed with S. pneumoniae supernatant containing the neuraminidase enzyme in a 1:1 ratio in a test tube and incubated at 37 °C for 24 hours.
Lectin Preparation
Lectin reagent from raw Arachis hypogaea seeds was prepared according to Judd’s method.11 The seeds were weighed, and 1.0250 gm was placed into 7.5 mL of phosphate-buffered saline (PBS) and soaked in the solution for 24 hours. The seeds were then ground into a paste using a mortar and pestle, including the 7.5 mL of PBS used for soaking the seeds. The test tubes were then filled with approximately one-third of the seed sediment and one-third of the PBS liquid. The tubes were centrifuged at 450 × g (1400 RPM) for 5 minutes, and the supernatant was removed and placed into a sterile container. A concentration of 0.1% sodium azide was added in a 1:1 ratio with the lectin to prevent microbial growth during storage.
Alsever’s Solution
Alsever’s solution is an isotonic saline liquid that can be used as an anticoagulant and preservative for in vitro storage of reagent RBCs. To create the Alsever’s solution, 0.55 gm of citric acid, 20.5 gm of dextrose, 2.0 gm of inosine, 4.2 gm of sodium chloride, and 8.0 gm of trisodium citrate were dissolved in 600 mL of distilled water. Once fully dissolved, 0.33 gm of chloramphenicol and 0.5 gm of neomycin sulfate were added and mixed. The solution was diluted to 1 L with distilled water and refrigerated at 1–8 °C for a maximum of 10 weeks. All materials other than the distilled water were purchased through Fisher Scientific.
Assay Validation
Blood was collected via venipuncture into a 2 mL EDTA test tube following approval from the Weber State University Institutional Review Board (IRB). The sample was collected from an O-negative donor. After activating the T antigen through the previously described procedure, the blood was transferred to a 12 × 75 mm borosilicate tube, washed 3 times in normal saline, and made into a 3%–5% cell suspension. The T-activated RBCs were used as the positive QC cells, and untreated type O-negative reagent RBCs (Immucor’s Screening Cell III) were used as the negative QC cells. A disposable pipette was used to add 2 drops of lectin to each of 2 clean, labeled test tubes, and 1 drop of each of the positive and negative QC cell suspensions was added to the appropriately labeled tubes. Both tubes were incubated at RT for 5 minutes and centrifuged at 1600 x g (3500 RPM) for 15 seconds. The strength of the hemagglutination reaction was recorded. After the reactivity of the lectin and T-activated cells and nonreactivity of the lectin and untreated cells were observed, the T-activated blood was separated into 6 equal aliquots. Alsever’s solution was added in a 1:1 ratio and was stored in the refrigerator at 1–8 °C.
The lectin and positive and negative QC cells were tested at each 4-week interval. To perform this monthly testing on the positive QC cells, 1 of the T-activated RBC aliquots was removed from the refrigerator. The RBCs were washed 3 times in normal saline to remove the Alsever’s solution and made into a 3%–5% cell suspension. Using a disposable pipette, 1 drop of each of the positive and negative RBC suspensions was added to 2 drops of lectin into 2 appropriately labeled test tubes. The tubes were incubated for 5 minutes at RT and centrifuged at 1600 × g (3500 RPM) for 15 seconds, and the strength of agglutination was recorded.
Survey Distribution
A survey was conducted following approval from the Weber State University IRB. The survey was prepared through Microsoft Office Forms and was sent via e-mail to 75 transfusion medicine and IRL medical laboratory scientists, laboratory managers, and PhD scientists using contact lists from America’s Blood Centers (ABC) and the Invitational Conference of Investigative Immunohematologists. The following ABC locations were sent emails: Blood Assurance, Blood Bank of Alaska, Blood Bank of Hawaii, Blood Center of Northcentral Wisconsin, Bloodworks Northwest, Carter BloodCare. Cascade Regional Blood Services, Central California Blood Center, Central Pennsylvania Blood Bank, Children’s Hospital Los Angeles, Coastal Bend Blood Center, Community Blood Bank of Northwest Pennsylvania & Western New York, Community Blood Center (Appleton), Community Blood Center (Dayton), Community Blood Center of the Ozarks, ConnectLife, Hema-Quebec, Gulf Coast Regional Blood Center, Houchin Community Blood Bank, Hoxworth Blood Center, Impactlife, Kentucky Blood Center, Lifeline Blood Services, Lifeserve Blood Center, LifeShare Blood Center, LifeSouth Community Blood Center, LifeStream, Medic Regional Blood Center, Miller-Keystone Blood Center, Mississippi Blood Services, New York Blood Center, Northern California Community Blood Bank, Oklahoma Blood Institute, OneBlood, Rock River Valley Blood Center, San Diego Blood Bank, Shepard Community Blood Center, South Texas Blood & Tissue Center, Stanford Blood Center, SunCoast Blood Centers, Texoma Regional Blood Center, the Blood Center (New Orleans), The Blood Connection, Versiti, Vitalant, We Are Blood, & Western Kentucky Regional Blood Center. The survey link was accessed by 15 individuals, 13 of which responded. The survey’s questions assessed current practices and procedures for testing PA.
RESULTS
To determine the long-term storage and stability of the assay reagents, testing was carried out periodically for 4 months. RBCs treated with neuraminidase (positive QC) and untreated cells (negative QC) were exposed to the isolated lectin reagent. The T-activated cells used for positive QC produced strong hemagglutination reactions (4+, on a scale of 0 to 4+), and the negative QC cells demonstrated no agglutination for 3 months (Table 1). In the beginning of the fourth month of testing, the T-activated cells were hemolyzed, and a 3%–5% cell suspension could not be created for testing.
Monthly hemagglutination reactions for positive and negative QC cells
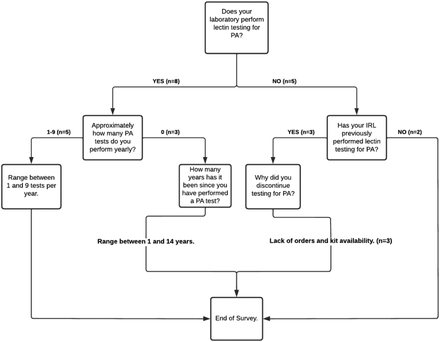
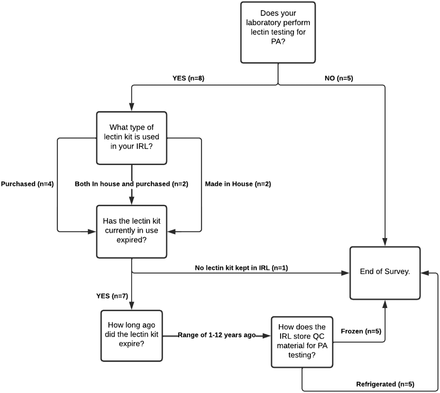
To determine the frequency and methods used to test for PA in the United States, a survey was administered, and the results were analyzed. The survey responses (Figure 1) indicated that the respondents occasionally encounter cases of PA, approximately 1 to 9 times each year, but must depend upon non–Food and Drug Administration–approved methods of testing to confirm such cases. Of those that perform testing for PA, 4 of the respondents indicated an in-house prepared lectin kit is used (Figure 2). Of the 8 laboratories that perform PA testing, 7 use expired reagents because the commercial manufacturers have discontinued their production, and they are no longer available for purchase. Of the 3 laboratories that used to perform testing for PA, 2 have discontinued testing due to lack of kit availability.
Survey questions—PA testing occurrences. PA, polyagglutination.
Survey questions—PA testing storage and expiration. IRL, immunohematology reference laboratory; PA, polyagglutination; QC, quality control.
DISCUSSION
The purpose of this study was to assess the current practices regarding PA testing in the United States, to evaluate the need for lectins and QC cells to perform this testing, and to develop a testing alternative for IRLs to use by providing additional details regarding the strain of bacteria, incubation time, and shelf life and potency of the lectin and preparation of QC cells that were not described in Judd’s Methods in Immunohematology,11 the AABB Technical Manual,12 and Mould’s procedure for T activation.13 We determined that this method for A. hypogaea lectin extraction and T activation of RBCs used for positive QC can be easily made in-house using reagents and supplies commonly found in most clinical laboratories, and the QC cells can be used for up to 3 months if stored in Alsever’s solution and kept in the refrigerator between 1–8 °C.
The survey responses indicate that current testing methods for PA are not standardized. Of the respondents that test for PA, all of the lectin kits being used have expired. Different combinations of purchased and in-house lectin kits are used, and the storage requirements are not standardized. If the AABB IRL Standards continue to require PA testing, this method can be used to test for the T-activated form of PA instead of using expired reagents.
It is important to note that although the survey results were extremely valuable to the study, a response rate of 17.33% was achieved (13 out of 75) after multiple reminder emails to complete the survey were sent. The low response rate was unfavorable and can be considered a weakness to the study.
In order to preserve the anonymity of the respondents, no specific professional demographic data were collected, and no delineation was made between survey respondents or geographic regions of the respondents within the United States. Future studies could include a larger sample size as well as demographic and geographic data.
The stability of T-activated RBCs can be variable, depending upon the strength of the neuraminidase enzyme produced by S. pneumoniae strains as well as the particular RBCs used for T activation. Further studies should evaluate T-activated RBCs’ stability to determine if the modification of various other carbohydrate antigens found on the RBC membrane decreases its overall stability and how this affects shelf life.
Moulds’s method13 requires a 1-hour incubation of RBCs for the neuraminidase enzyme to activate the T antigen. However, when we tested the cells at the 1-hour interval, no agglutination was observed. After failed attempts with 1-, 2-, and 3-hour incubations, we successfully developed T-activated cells with a 24-hour incubation period. In further studies, the cells could be tested hourly during incubation at 37 °C to determine the point at which T activation can be reliably achieved.
It is important to note that additional steps in Judd’s method for lectin preparation11 were adjusted in order to achieve our results. For instance, Judd’s method does not specify the centrifugation time or RPM requirement stated when preparing the lectin reagent. In addition, our method requires incubation of the RBCs in the neuraminidase solution at 37 °C for 24 hours. Lastly, there was no specified RT incubation time when performing the monthly lectin testing. There are no considerable differences between the method for preparing lectins described in Judd’s Methods for Immunohematology11 and in the AABB Technical Manual,12 except Judd’s procedure requires the seeds to be soaked in PBS prior to grinding, and the AABB Technical Manual recommends incubating the ground seeds in saline for 4–12 hours. Thus, IRLs can use our updated version of either Judd’s method or the procedure in the AABB Technical Manual11,12 to produce lectin reagents.
Polyagglutinable RBCs should be tested against a panel of several different lectins to identify the form of PA because some lectins can bind to more than 1 cryptantigen. Additional studies should identify methods to reliably expose other cryptantigens, such as Tk, and verify the shelf lives of those lectins and QC RBCs.
Our survey data illustrate the need for a reliable method to test for PA because the IRLs that continue to perform PA testing use expired reagents. Our testing results demonstrate that this method can be used to test for the T-activated form of PA using equipment, supplies, and reagents found in most clinical laboratories. In this method, a potent lectin can be extracted, and positive QC cells can be produced and stored between 1–8 °C for up to 3 months.
Acknowledgements
The authors would like to thank the Weber State University Office of Undergraduate Research and the Kem and Carolyn Gardner Foundation for funding this research. We also wish to thank Dr Sue Harley, Botany Department Chair, for sharing her lectin expertise and Dr Edward Walker for sharing his knowledge of enzyme function in biological systems. Lastly, we wish to thank Nicholas Quesnell and Alyson Stanger for their assistance with bacterial growth and the Medical Laboratory Sciences Department for the use of facilities.
- Received June 3, 2022.
- Accepted February 16, 2024.
American Society for Clinical Laboratory Science