This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Bethany N. Kent, PhD, MLS(ASCP)CM⇑
- Address for Correspondence: Bethany N. Kent, PhD, MLS(ASCP)CM, PathGroup Labs, LLC, 658 Grassmere Park, Nashville, TN 37211, 615-562-9480, bkent{at}pathgroup.com
Compare the symptoms of bacterial and protozoal sexually transmitted diseases.
Describe the threat of emerging antibiotic resistance for bacterial sexually transmitted diseases.
Compare the benefits of molecular diagnostic testing to traditional culture methods of identification.
Abstract
Bacterial and protozoal sexually transmitted infections (gonorrhea, chlamydia, syphilis, trichomoniasis) are curable, even though they remain as some of the most prevalent sexually transmitted diseases (STDs) worldwide. After a period of decreased infection rates, the reported instances of infection of this group of STDs is on the rise. Although widely accepted treatment is available for each of these diseases, gonorrhea in particular is beginning to demonstrate a pattern of antibiotic resistance that is predicted to lead to future treatment difficulties. Diagnostic capabilities have greatly increased the rapidness of treatment as recent technological advances in molecular methods has significantly impacted the speed and accuracy of STD diagnosis.
ABBREVIATIONS: CDC - Centers for Disease Control, STD - Sexually Transmitted Disease, STIs - Sexually Transmitted Infections, PID - Pelvic Inflammatory Disease, HIV - Human Immunodeficiency Virus, HSV - Herpes Simplex Virus, GISP - Gonoccocal Isolate Surveillance Project
INTRODUCTION
The Centers for Disease Control (CDC) considers gonorrhea, chlamydia, and syphilis, (caused by the gram-negative diplococcus Neisseria gonorrhoeae, the obligate intracellular bacteria Chlamydia trachomatis, and the spirochete Treponema pallidum, respectively) to be reportable organisms. Therefore, there is a significant amount of statistical analysis of surveillance information available on these organisms and the sexually transmitted infections (STIs) that they cause. These three organisms remain the most common bacterial sexually transmitted infections; when also including the protist trichomonas, this group is responsible for 357 million infections per year.1 These infections are the only curable examples within the most prevalent STDs. While gonorrhea and chlamydia are curable, infection can lead to permanent infertility if inflammation of the endometrium, fallopian tubes, ovaries, and pelvic cavity, known as Pelvic Inflammatory Disease (PID), occurs.2 PID is a disease that affects over 1 million women in the US at a cost of roughly $2.7 billion.3
Gonorrhea
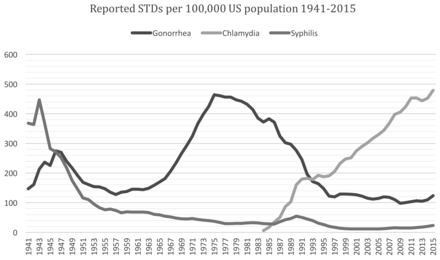
Gonorrhea is believed to be the oldest recognized STD/STI, with mentions dating back to the Old Testament in Leviticus.4 Socially it was known as a “venereal” disease by the 13th century and epidemiologically it was the most prevalent, and thus most well understood STD/STI for many decades. Microbiologically it was thought to be the first phase of Syphilis until 1850, but Albert Neisser, for whom it is named, later distinguished it as a unique infection in 1879.4 The era of antimicrobials made gonorrhea historically one of the easiest STD/STIs to treat since sulfonamides, penicillin, and tetracyclines were proven effective against 80-90% of infections in the 1930s-1940s. However, Neisseria gonorrhoeae has shown the robust ability to mutate to form antibiotic resistant strains.5 The case rate in the U.S. has ebbed and flowed over the 80 years that it has been tracked; recently, a period of increase has been noted to approximately 124 cases/100,000 population.6 (Figure 1)
N. gonorrhoeae, which only infects humans, can persist in the urogenital tract, the anus, the eye, or the pharyngeal tract. In some cases, it can lead to a systemic infection and cause joint inflammation or meningitis. Transmission occurs through direct contact or contact with infected body fluids.6 Neisseria gonorrhoeae is a fastidious organism that is dependent on abundant moisture, enriched nutrients and high levels of CO2 to sustain microbial life.7 Initial clinical laboratory evidence of an infection is the presence on a Gram stain of white blood cells having phagocytized the coffee-bean shaped diplococci that give N. gonorrhoeae the name “gonococcal.” If the gonococci survive the initial onslaught of the immune system, an incubation period of 1-14 days (average 2-5 days) occurs prior to the appearance of symptoms that affect primarily the skin and mucous membranes. While both men and women can be asymptomatic, men are more likely to exhibit symptoms.8,9 Congenitally, N. gonorrhoeae can also infect the eyes of newborns during birth if the mother has a current infection. Symptoms of infection include genital discharge that can be white, green, or yellow, burning while urinating, and itching.10
Treatment
Gonorrhea, like many of the prevailing infections of the modern era, has developed a steady resistance pattern to antimicrobials used for treatment. The Gonococcal Isolate Surveillance Project (GISP) was activated in 1986 to monitor trends in gonococcal antimicrobial resistance.11 Due to the fear of the emergence of an untreatable gonococcal strain, current recommendations involve dual-antibiotic treatment with ceftriaxone and azithromycin instead of treating with ceftriaxone alone.11,12 This treatment strategy may be short-lived, however, as resistance to these drugs of choice is already appearing with regularity. The risk of congenital infection is mitigated by prophylactic therapy that consists of erythromycin eye ointment given to an infant shortly after birth.
Prevalence of reportable bacterial STDs Gonorrhea, Chlamydia, and Syphilis. After over a decade of decline for gonorrhea and syphilis, rates are beginning to rise. Chlamydia demonstrates steady increase in rates of infection to become the most prevalent bacterial sexually transmitted disease.6
Chlamydia
Infections caused by Chlamydia trachomatis are among the most prevalent of all sexually transmitted diseases.12 (Figure 1) In women, these infections often result in serious reproductive tract complications, such as PID, infertility, and ectopic pregnancy.13 The long-term effects of infection is compounded by the frequency (70-90%)14 of asymptomatic infection. Additionally, infected pregnant women can pass the infection to their infants during delivery. Chlamydia became a CDC-reportable disease in 1984 and a combination of increased infections and more sensitive screening techniques have led to a demonstrable increase in reported infections. In 1994, the 448,984 reported cases of chlamydia exceeded gonorrhea for the first time since surveillance began.15 Rates of infection continue to climb steadily; the current rate is 478 per 100,000 in the U.S. (Figure 1).
C. trachomatis infection is easily treatable and is not demonstrating the same quickly evolving antibiotic resistance trends seen in N. gonorrhoeae. Recommended treatment for chlamydial infections include azithromycin or doxycycline.16 Failed treatment, which occurs in 34% of US cases per year, is most commonly due to reinfection as opposed to resistance.17
Gonorrhea and Chlamydia Detection
Detection methodologies for N. gonorrhoeae and C. trachomatis have shifted over the last several decades. Culture was the gold standard until technology advanced in the realm of nucleic acid testing to the degree of 25-35% more sensitivity. The CDC updated recommendations for laboratory-based testing in 201418 and now prefer the use of nucleic acid testing (NAT) over other methods for symptomatic adults. This preference is due to the difficulty in keeping these fastidious organisms viable during transportation and in the technical expertise required to culture C. trachomatis as it is an obligate intracellular organism that requires host cells to survive. However, culture is the required method for children because NAT methods have not been sufficiently validated for those populations.
Syphilis
Syphilis has a long history as a sexually transmitted disease as it was first recognized in 15th century Europe.19 The causative agent of syphilis is the spirochete Treponema pallidum and it can be transmitted sexually or congenitally. Humans are the only known hosts and transmission is virtually always by direct contact with infectious lesions, generally through sexual contact.20 The CDC estimates that 74,702 cases of syphilis were diagnosed in 2015 with the highest incidence of primary infections (81.7%) occurring in men who have sex with men.21
Syphilis occurs in multiple different stages with different characteristics and symptoms. The incubation period lasts from 10-90 days with an average of 21 days. Once symptoms appear, there are four stages: the primary stage, the secondary stage, the latent stage, and the tertiary stage. The initial lesion, or chancre, appears at the point where the bacteria entered through a break in intact skin or mucous membrane. Multiple chancres appear if organism enters at different sites. In its typical presentation, the chancre presents as a clean, painless, indurated ulcer but there may be several variations in presentation. Common sites for the lesions include genitalia, rectum, urethra and mouth.
The primary lesion (chancre) lasts for 3 to 8 weeks and usually is accompanied by regional adenopathy. This lesion may disappear on its own but treatment is needed to prevent the disease advancing to secondary syphilis. A clinical finding that accompanies the chancre are lymph nodes that are large, round, discrete, rubbery, freely movable and usually non-tender. When the chancre first occurs, in most cases it is too early for serology to become positive, so a dark field microscopic examination can be performed for diagnosis.22
Secondary syphilis is distinguished by a rough, red, non-itchy rash on the palms of the hands and the soles of the feet that begins approximately 10 weeks after the initial appearance of the chancre. Lesions known as Condylomata lata may be evident in the mouth, underarms, or groin. These lesions are moist, flat, bilaterally symmetric, non-indurated lesions with high concentrations of Treponema. Other symptoms such as malaise, headache, fever, and generalized lymphadenopathy may be evident.22
Latent syphilis is by definition the stage in which there is a positive serological test for syphilis in the absence of any clinical disease symptoms. Its duration is highly variable. Approximately 25% of patients experience a relapse of secondary syphilis but only about 1/3 of latent cases progress to tertiary syphilis 1-40 years after initial infection.23
The disease process of congenital syphilis begins at 18 to 20 weeks of gestation, when the immune system is capable of responding. The disease is systemic and does not go through the primary syphilis stage. A stillborn fetus may result, and for those babies that have onset at delivery, the prognosis is poor.24 Those who survive usually present with the disease a few weeks after delivery.21 The rate of congenital syphilis is increasing in the U.S.; between 2012 and 2014, the rate increased from 8.4 to 11.6 cases per 100,000 live births.25
Detection
Detection of syphilis can be performed using tests specific for T. pallidum or tests that are non-specific for T. pallidum. Screening tests are typically non-specific tests because they detect the presence of anti-cardiolipin rapid plasma reagin (RPR) antibody that can be present due to other infectious diseases such as malaria, or autoimmune disorders26 and the sensitivity is approximately 75%.19 Once a screening test is positive, a more specific confirmatory test is performed using agglutination or fluorescent antibody absorption to detect the presence of T. pallidum. The expense of the confirmatory test has led to treatment for syphilis without knowing if that is the cause of the presented illness.27 The FDA approved the first point-of-care test for syphilis in 2014 but studies have shown that it has a low sensitivity of ~71%.28
Treatment
T. pallidum is sensitive to Benzathine penicillin G and a single intramuscular injection can cure the disease in any stage except late latent syphilis. For the late latent stage, three shots weekly is given until the infection is cleared.29
Trichomoniasis
Trichomoniasis, transmitted by the protist Trichomonas vaginalis, is the most common STD worldwide that is not of viral origin, with an estimated 8.1% of women infected.30 However, since the CDC does not require reporting of T. vaginalis infections, the propensity of infection in the United States is not known. Although the large majority of both men and women are asymptomatic, approximately one-third begin to show symptoms after six months of infection 31 while infection can be self-limiting within two weeks in males.32 Infected women are typically cleared of infection by treatment with metronidazole or tinidazole.33
Symptoms of T. vaginalis infection, which infects the squamous epithelial cells of the genital tract, include yellow-grey-green discharge, vaginal itching, foul odor 34 and a “strawberry cervix”. 35 While the overt symptoms are not dangerous, infection with T. vaginalis has secondary consequences. An infection during pregnancy has been linked to premature birth at a rate of 1.4 times the uninfected rate.36 Infection with T. vaginalis also increases the risk of becoming infected with other sexually transmitted infections, especially Human Immunodeficiency Virus (HIV) and Herpes Simplex Virus (HSV); one study attributes 6.2% of female HIV infections in the US to T. vaginalis co-infection.37 There are three suspected reasons for this increase in vulnerability to HIV infection: 1) the immune response to T. vaginalis recruits HIV target cells to the genital area, 2) infection destroys mucosal cells in the genital tract via mucosal hemorrhages, and 3) an alteration in normal vaginal flora makes the genital tract more tolerant to HIV infection.33 T. vaginalis not only makes females more susceptible to infection but it also increases the ability to transmit the virus to a sexual partner by increasing the amount of viral shedding in vaginal discharge.38
Diagnostic Testing
The quickest diagnostic test for T. vaginalis is the microscopic wet preparation from a vaginal swab. The appearance of motile, flagellating cells with an undulating membrane under 40X power is sufficient for diagnosis.38 A caveat to using a wet mount for diagnosis is that the T. vaginalis cells die quickly once outside the genital tract but a living cell must be observed for the test to be called positive. Cultures are available but not widely used as they are expensive, have poor sensitivity, and have a long turn-around time of up to 7 days.33 One high sensitivity point-of-care test is FDA approved and yields results in 15 minutes but it lacks the advantage of being able to detect other reasons for symptoms of genital infection. Nucleic acid testing has become the preferred method as it is highly sensitive, has a fast turn-around-time and most platforms combine T. vaginalis testing with other sexually transmitted infections or causes of bacterial vaginosis.38
CONCLUSION
The bacterial and protozoal sexually transmitted diseases are widespread but treatable with widely available antimicrobials. However, threats of antimicrobial resistance are becoming more realized with the new emergence of gonococcal resistance isolates, which will significantly change the landscape of sexually transmitted diseases worldwide. One important tool in the battle against the increase in infections is the rapid diagnosis that molecular testing has allowed. Further advances in molecular diagnostics could lead to rapid typing of infectious strains and their resistance patterns.
- © Copyright 2017 American Society for Clinical Laboratory Science Inc. All rights reserved.