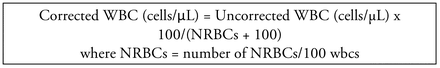This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Kathy Doig, PhD, MLS(ASCP)CM SH(ASCP)CM⇑
- Leslie A. Thompson, MS, MLS(ASCP)CM
- Address for Correspondence: Kathy Doig, PhD, MLS(ASCP)CM SH(ASCP)CM, 354 Farm Lane, Rm. N322, E. Lansing, MI 48824,517-353-1985, doig{at}msu.edu
List the white blood cell parameters of the complete blood count.
Describe the principle of analysis for each of the white blood cell parameters of the complete blood count.
Recognize instances in which the white blood cell count may be spurious and require technical or mathematical correction before reporting.
Apply appropriate techniques to spurious white blood cell counts to achieve valid counts.
Given relative differential counts and total white blood cell count, calculate absolute white blood cell differential counts.
Use proper terminology to describe elevations and decreased of white blood cell numbers and differential cell counts.
Given the white blood cell parameters of a complete blood count, apply a methodical approach to interpretation of the results for diagnostic and clinical purposes.
Abstract
Consistent use of a methodical approach to interpreting complete blood count (CBC) results can help detect spurious results that require remedy before results are reported. It can also help ensure that no clinically important information is overlooked during diagnostic interpretation of the results. The steps for interpreting the white blood cell portion of the CBC are:
Ensure that nucleated red blood cells or other conditions are not falsely affecting the white blood cell count (WBC); correct the WBC if needed, before proceeding.
Examine the WBC for variations in the total number of white blood cells.
Interpret absolute differential counts against appropriate reference intervals using proper terminology.
If absolute counts are not available from an instrument, use relative counts (i.e. percentages) to calculate absolute values.
Make note of immature cells in any leukocyte cell line reported in the differential that should not appear in normal peripheral blood.
Make note of any morphological abnormalities of wbcs.
Correlate the wbc findings with red blood cell and platelet findings for a complete clinical assessment of the patient's blood picture.
Explanations for conducting the evaluations are provided and the above steps are applied to example cases to demonstrate how this approach is used to interpret the wbc parameters of the CBC.
ABBREVIATIONS: CBC - complete blood count, dL-deciliter, fL-femtoliter, g-gram, NRBC-nucleated red blood cell, rbc-red blood cell, RNA-ribonucleic acid, wbc-white blood cell, WBC-white blood cell count, μL-microliter
INTRODUCTION
Though a complete blood count (CBC) is ordered as a single test, it is a battery of multiple tests and calculations that collectively assess the cellular elements of the blood: red blood cells (rbcs), white blood cells (wbcs) and platelets. Clinical interpretation of the CBC requires that all results be evaluated thoroughly since some patient conditions will affect all cell lines. However, it is helpful to review the multiple results for each of the cellular elements separately, correlating within that group of tests, before a comprehensive assessment is completed. This article will focus on a method for interpreting the results of the wbc-related assays.
The method recommended here differs from commonly published diagnostic algorithms in that it incorporates strategies for laboratorians to detect invalid results so they can be corrected before clinical interpretation. Furthermore, there is an emphasis on translation of numerical data into a narrative description that is more easily communicated. This is especially helpful for laboratory science students since it does not make prior assumptions about the reader's vocabulary.
The sequencing of the steps below is not necessarily fixed. However, the consistent use of Step 1 as the first step ensures that the white blood cell count (WBC) is accurate and not falsely affected by spurious results. With an inaccurate WBC, all other conclusions that follow will be inaccurate. Many modern instruments will recognize and automatically correct for some interferences so that an accurate WBC can be reported. Once an accurate WBC is assured, evaluating the total WBC (Step 2), is a sensible starting place for thorough interpretation of the results. The steps that follow are then logical. It is important that no parameter is overlooked since the reported parameters provide related, but different, information for quality control assessment and patient diagnosis. The white blood cell portion of the CBC includes the WBC and the wbc differential count (relative and absolute values, and morphological evaluation, if justified). The steps to interpreting the WBC are presented in Table 1.
Using the Steps for the Interpretation of White Blood Cell Parameters
Step 1. Ensure that nucleated red blood cells (NRBCs) or other conditions are not falsely affecting the WBC; correct the WBC if needed, before proceeding.
Steps for interpreting the white blood cell parameters of a complete blood count.
The lysing solution used in WBC counting on some instruments removes rbcs leaving only nucleated cells for counting. Under normal circumstances, only WBCs remain intact for counting. However, NRBCs also resist lysis and thus are counted with the wbcs when present, falsely elevating the WBC. Modern instruments are able to identify NRBCs and subtract them from the WBC value, thus ensuring that the WBC represents only wbcs, as intended. In instances when an instrument-corrected WBC value is not available, a manual correction must be applied.
The method for manual correction of a WBC involves counting the number of NRBCs encountered while conducting a microscopic 100-cell wbc differential count. That value is reported as NRBCs/100 wbcs. Most laboratories will not correct the WBC until the number of NRBCs encountered creates a meaningful difference in the WBC. Thus, the protocols typically direct that the WBC be corrected when there are, for example, 10 or more NRBCs/100 wbcs, thus allowing for up to a 10% error in the count. More stringent limits can be set and are particularly important for lower WBCs.1 Institutional protocol should be followed in all instances.
The WBC must be corrected for NRBCs when two conditions are met: 1) the differential was generated manually OR if the instrument cannot correct the count and 2) the number of NRBCs reported is at or above the threshold of the institutional protocol. The formula for correcting the WBC for the presence of NRBCs is described in Figure 1. The derivation of the formula is available in Table 2. The corrected WBC value, whether instrument or manually generated is used to make all additional wbc assessments.
The formula for correcting the WBC for the presence of NRBCs is shown
Derivation of the formula for correction of the white blood cell count due to NRBCs.
Other causes of false values must also be detected and corrected.2 Falsely elevated WBC values may be due to aggregated platelet clumps or incomplete rbc lysis. Instruments are typically able to flag these, though they may not be able to correct for them automatically. In the case of aggregated platelets, the clumping increases the size of particles to be comparable to wbcs. In this situation, a simultaneous pseudothrombocytopenia is also expected. A redraw into a blue top sodium citrate tube is necessary and then the measured WBC value must be multiplied by 1.1 to adjust for the anticoagulant dilution. In the case of incomplete rbc lysis that can occur in conditions such as thalassemia and sickle cell anemia, rbcs will remain in the WBC dilution fluid and be counted as wbcs. Preparing a manual dilution and allowing it to incubate longer before instrument sampling should permit the cells to lyse, thus providing an accurate WBC.
Falsely low WBC values, called pseudoleukopenia, may occur with cryoglobulins that agglutinate the wbcs3 and may warrant an instrument flag due to their very large size. Warming the sample before testing may provide an accurate count. In other cases, a redraw into sodium citrate may be necessary and the instrument generated WBC will need to be multiplied by 1.1 before reporting. Some patients' wbcs will clump in the presence of EDTA without cryoglobulins being present and a redraw into citrate will correct the problem.4 In instances where the instrument may not be able to flag these false values, examination of the blood film (see Step 6) may identify them and prompt corrective action. The remaining steps should be completed once an accurate WBC is available.
Step 2. Examine the WBC for variations in the total number of wbcs.
WBC values vary depending on age, particularly in the early months of life, through childhood, and into adolescence.5 While gender differences are not observed, the WBC can vary with genetics, typically as a result of differences in specific cell lines.6 Thus, the use of the proper reference interval is especially important to the interpretation of WBC values. An increase above the reference interval is called leukocytosis while a decrease is leukopenia.
Step 3. Interpret absolute differential counts against appropriate reference intervals using proper terminology.
Like the total WBC, the quantity of the subtypes of wbcs that are normally present depends on age.5 Benign racial differences are also documented.6 Thus, appropriate reference intervals are essential. The absolute wbc differential enumerates the number of each subtype of wbc that the instrument is able to distinguish. When reported as cells/volume, e.g. neutrophils/μL, the number is called an absolute value because it is an actual concentration of that cell type. The alternative is the relative value expressed as a percentage and subject to misinterpretation (discussed below). Modern instruments actually count absolute values and calculate relative differential values (Figure 2). The absolute differential values are the best value to use in making diagnostic conclusions, especially when generated by an instrument.
The calculation of relative differential values is shown
To interpret absolute differential counts, follow these steps.
As a quality check, add up the absolute counts of the subtypes of cells and it should equal the total WBC.
For a given cell line, add together all cell counts of any stage, e.g. add together all the stages of neutrophilic cells (promyelocytes + myelocytes + metamyelocytes + bands + neutrophils), and evaluate the total relative to the reference interval for the mature form of that cell, since that interval represents the number of those cells normally expected (for neutrophils, the interval can include the upper end of neutrophils plus bands).
Use the following terms to describe the value:
Increases above the top of the reference interval include: neutrophilia, lymphocytosis, monocytosis, eosinophilia, basophilia.
Decreases below the bottom of the reference interval: neutropenia and lymphopenia. Monocytopenia, eosinopenia, and basopenia are not typically noted since the lower limits of the normal reference intervals are so low
Step 4. If absolute counts are not available from an instrument, use relative counts (i.e. percentages) to calculate absolute values.
Absolute differential values will not be available when the differential is generated microscopically; only the percentage of each cell type is known. In this case, because the number of cells counted is limited to 100, the number of cells of each type observed within that 100 cell limit is relative to the frequency of each of the other cell types. Misinterpretation occurs when, for example, the concentration of lymphocytes actually present in the sample is normal, but the concentration of neutrophils increases during a bacterial infection. As the relative (i.e. manual) differential is conducted, neutrophils are encountered and counted more frequently, thus reducing the number of lymphocytes that will be encountered and counted within 100 cells. The lymphocytes will appear to be reduced relative to the neutrophils, though in fact, they are normal. It is this risk of misinterpretation of the relative differential that limits its value.
Absolute values can be calculated from the relative differential percentages for more accurate interpretation, as long as the total WBC is known (Figure 3). One can assume that the absolute counts are normal if the total WBC is within the reference interval and all the relative values are within their reference intervals. In such instances, calculation of absolute values is not necessary. Conversely, absolute counts should be calculated for all cell types, not just those outside relative reference intervals, when 1) the total WBC is outside the reference interval (low or high) OR 2) any relative value is outside its reference interval. After calculating absolute values, follow the steps for interpreting absolute values described in step 3.
The calculation of absolute values from relative differential percentages is shown.
Step 5. Make note of immature cells in any leukocyte cell line reported in the differential that should not appear in normal peripheral blood.
Young cells of any leukocyte cell line should have been noted when interpreting the differential e.g. prolymphocytes plus lymphocytes contributing to a conclusion of lymphocytosis. However, the presence of young cells that are abnormal typically carries serious clinical implications, so even small numbers must be noted. To continue the example, perhaps the prolymphocytes are too few to contribute to overall lymphocytosis. Nevertheless, they cannot be ignored.
Two additional items pertinent to young cells merit mention. 1) Elevation above reference values for bands or any of the younger neutrophilic cells is called a “left shift.” This phrase is used exclusively to describe the presence of young neutrophilic cells. The origin of the term “left shift” refers to frequency distribution histograms labeling the stages from left to right with the youngest on the left. Generating such graphs was a standard practice historically. When young cells were present, the distribution histogram shifted left.7 2) NRBCs may require correction of the white blood count; refer to Step 1 above.
Step 6. Make note of any morphological abnormalities of wbcs.
Typically, when the wbc morphology is normal, there is no notation in the report; only abnormalities are reported. Morphological abnormalities of wbcs may affect the overall cell appearance (i.e. reactive lymphocytes), just the nucleus (i.e. hyper- and hyposegmentation, multiple nuclei, nuclear blebbing, Reider forms), or just the cytoplasm (i.e. toxic granulation, vacuolization, agranularity, cytoplasmic blebbing).
When performing the morphological assessment, it should be standard practice to perform an estimate of the WBC as a quality check, helping to ensure that the slide being examined is from the same sample as the numerical parameters. The estimate is expected to match the measured value. However, if the estimated value and the instrument value do not correlate well, it may indicate a spurious instrument result.
Estimation of the WBC is done by observing 10 fields with 40× or 50× objective and averaging the number of white blood cells in those fields.7 Multiply the average obtained with a 40× objective by 2 × 103/μL or multiply the average using 50× objective by 3 × 103/μL. The result should approximate the instrument value, assuming the patient has a normal rbc count and the optimum area of the slide is used in this assessment.
Step 7. Correlate the wbc findings with rbc and platelet findings for a complete clinical assessment of the patient's blood picture.
Application of the Steps to the Interpretation of Results for Sample Cases
Case 1: The WBC and differential results presented in Table 3 are for a 6-year-old African-American girl. The testing was conducted in the physician office using an instrument that provides a 5-cell relative differential. From the information given, one can use the 7 steps to assess this patient's white blood cell picture.
Step 1. Ensure that NRBCs or other conditions are not falsely affecting the WBC; correct the WBC if needed, before proceeding.
Looking at the white blood cell picture, the sample likely flagged the instrument and thus reflexed a manual differential. During this microscopic review, it was noted that the patient had 14 NRBC/100 wbcs, which is significant in affecting the validity of the WBC determined by the instrument. A correction calculation must be made.
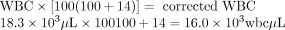
Therefore, all subsequent assessments are conducted on a WBC of 16.0 × 103 wbc/μL
White blood cell parameters for Case 1.
Step 2. Examine the WBC for variations in the total number of wbcs.
16.0 × 103 WBC/μL is above the pediatric reference interval upper limit, thus, there is a leukocytosis.
Step 3. Interpret absolute differential counts against appropriate reference intervals using proper terminology.
Since absolute values are not generated by this instrument, proceed to Step 4.
Step 4. If absolute counts are not available from an instrument, use relative counts (i.e. percentages) to calculate absolute values. See Table 4 for the calculated absolute counts using the formula in Figure 3.
The patient has an absolute neutrophilia while the lymphocytes are within the normal reference interval, as are the monocytes.
To verify the calculations of the absolute values, total them; (9.6 + 5.3 + 1.1) × 103/μL = 16.0 × 103/μL which was the total WBC. Therefore, the calculations are correct.
Calculations of absolute white blood cell values for Case 1.
This patient's relative values demonstrate why using absolute values is important to the proper interpretation of differentials. Adding the bands and neutrophils to a total of 60% leads to the conclusion that this patient has a relative neutrophilia. This matches the conclusion drawn from the absolute neutrophil assessment. The relative lymphocyte value is below the lower limit of the reference interval. While a relative lymphopenia exists, note that the absolute value for lymphocytes was within the reference interval; therefore, no lymphopenia is present. Furthermore, a relative monocytosis is noted but the absolute value is within the reference interval. Thus, using the relative values alone for this patient would lead to faulty conclusions about the numbers for lymphocytes and monocytes.
Step 5. Make note of immature cells in any leukocyte cell line reported in the differential that should not appear in normal peripheral blood.
This patient has 15% bands (2.4 × 103/μL) noted in her differential, and this would be described as a left shift. No other immature cells are noted.
Step 6. Make note of any morphological abnormalities of wbcs.
This patient's neutrophils are showing vacuolization and toxic granulation. During a microscopic differential using 50× objective, an average of 5-6 white blood cells / field should be encountered to correlate to a WBC of 16.0 × 103/μL.
Step 7. Correlate the wbc findings with rbc and platelet findings for a complete clinical assessment of the patient's blood picture.
In summary, this patient's wbc picture is described as leukocytosis with an absolute neutrophilia, a left shift, and toxic changes to the neutrophils. This picture is one of infection or inflammation. The presence of NRBCs suggests a significant anemia, which was seen with a low hemoglobin (rbcs values not shown). The rbc morphology included anisocytosis, poikilocytosis, sickle cells, target cells, and polychromasia. Her platelet values were all within normal limits. This girl had gone to the physician experiencing severe abdominal pain under her ribs on the left side, likely related to the spleen, as well as fever. Sickle cell patients are prone to infection due to diminished spleen function.
Case 2: The WBC and differential presented in Table 5 were ordered by a physician in the emergency department on this 53-year-old man. From the information given, one can use the 7 steps to assess this patient's white blood cell picture.
White blood cell parameters for Case 2.
Step 1. Ensure that NRBCs or other conditions are not falsely affecting the WBC; correct the WBC if needed, before proceeding.
There are no NRBCs reported or other flags generated, so the WBC is reliable as reported.
Step 2. Examine the WBC for variations in the total number of wbcs.
19.3 × 103 WBC/μL is above the adult reference interval upper limit, so there is a leukocytosis.
Step 3. Interpret absolute differential counts against appropriate reference intervals using proper terminology.
Comparing the absolute values provided to their reference intervals, the patient has an absolute neutrophilia when the neutrophils and bands are combined, a slight absolute lymphocytosis, an absolute monocytosis, an absolute eosinophilia and an absolute basophilia.
Step 4. If absolute counts are not available from an instrument, use relative counts (i.e. percentages) to calculate absolute values.
Absolute values were provided by the instrument and interpreted in Step 3; proceed to
Step 5. Make note of immature cells in any leukocyte cell line reported in the differential that should not appear in normal peripheral blood.
No immature cells were noted nor was a left shift present.
Step 6. Make note of any morphological abnormalities of wbcs.
Nothing abnormal is noted. During a microscopic differential using a 50× objective, an average of 6-7 wbcs should be seen in each field to correlate to the WBC of 19.3 × 103/μL.
Step 7. Correlate the wbc findings with rbc and platelet findings for a complete clinical assessment of the patient's blood picture.
In summary, this patient's wbc picture was one of leukocytosis with elevations in all cells lines but no abnormal morphology or immature cells noted. The elevations of all cell types may point to dehydration, particularly since no morphological abnormalities or immature cells were noted. This patient was known to have alcoholic liver disease. He came to the emergency department due to generalized itching which can result from deposition of bile salts in the skin. His rbc parameters (not shown) demonstrated a mild macrocytic anemia with target cells and polychromasia, consistent with alcoholic liver disease. His platelet parameters (also not shown) were all normal. Rehydration might be expected to bring the WBC vales back to normal while revealing a more significant anemia.
Case 3: A CBC with differential were ordered by a physician in the emergency department on a 68-year-old male. See results in Table 6. From the information given, one can use the 7 steps to assess this patient's white blood cell picture.
White blood cell parameters for Case 6.
Step 1. Ensure that NRBCs or other conditions are not falsely affecting the WBC; correct the WBC if needed, before proceeding.
There were no NRBCs reported or other apparent interferences, so the WBC is reliable as reported.
Step 2. Examine the WBC for variations in the total number of wbcs.
45.0 × 103 WBC/μL is above the adult reference interval upper limit, so there is a leukocytosis.
Step 3. Interpret absolute differential counts against appropriate reference intervals using proper terminology.
This sample generated a flag for immature granulocytes and blasts. Thus, a manual differential was performed and absolute values were not available from the instrument.
Step 4. If absolute counts are not available from an instrument, use relative counts (i.e. percentages) to calculate absolute values.
Calculations for this patient are shown in Table 7. The patient has an absolute neutrophilia, even without counting the blasts. However, it should be noted that since Auer rods were noted in the blasts, they are likely neutrophilic, and if included in the relative totals, the neutrophilia is even greater. The patient's lymphocytes are within the reference interval as are the monocytes.
Calculations of the absolute differential counts for Case 3.
Although the eosinophils are technically below the reference interval, thus eosinopenia, this is not typically considered significant. The basophils are within the reference interval and since none were observed, no calculation is required.
Checking the calculations by adding up the absolute counts results in (41.9 +2.7+0.45) × 103 cells/μL = 45.05 × 103 cells/μL; thus, the individual cell values are correct.
Step 5. Make note of immature cells in any leukocyte cell line reported in the differential that should not appear in normal peripheral blood.
The patient has a drastic left shift, containing myelocytes, promyelocytes, and blasts.
Step 6. Make note of any morphological abnormalities of wbcs.
Auer rods were noted in the blasts. During a microscopic differential at 40×, an average of 22-23 white blood cells would be expected per field to correspond to the WBC of 45.0 × 103/μL.
Step 7. Correlate the wbc findings with rbc and platelet findings for a complete clinical assessment of the patient's blood picture.
In summary, this patient's wbc results include a leukocytosis due to a dramatic neutrophilia including a left shift with blasts. The other portions of the CBC (not shown here) demonstrated a normocytic, normochromic anemia with a few shistocytes and thrombocytopenia. He had visited the emergency department due to gastrointestinal bleeding likely related to the thrombocytopenia and/or low level of disseminated intravascular coagulation. Ultimately, he was diagnosed with acute promyelocytic leukemia.
SUMMARY
The systematic application of the steps detailed here will help ensure that reported results are accurate. Additionally, it will help ensure than no results are overlooked in the diagnostic evaluation of white blood cell parameters.
- © Copyright 2017 American Society for Clinical Laboratory Science Inc. All rights reserved.