This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Blood Bank Department, Frederick Memorial Hospital; The George Washington University School of Medicine and Health Sciences, Ashburn, VA
- Department of Biomedical Laboratory Sciences, The George Washington University School of Medicine and Health Sciences, Ashburn, VA
- Department of Biomedical Laboratory Sciences, The George Washington University School of Medicine and Health Sciences, Ashburn, VA
- Address for Correspondence: Marcia Firmani
, Department of Biomedical Laboratory Sciences, The George Washington University School of Medicine and Health Sciences, Ashburn, VA, firmanim{at}gwu.edu
ABSTRACT
In 2012, Frederick Memorial Hospital (FMH) revitalized their blood utilization guidelines to follow a more restrictive approach to blood product transfusions. With help from the American Red Cross and the American Association of Blood Banks, the blood utilization committee at FMH established a set of blood transfusion guidelines and educated and enforced all ordering physicians to follow the new set of guidelines. Since the guidelines were established, the amount of red blood cell transfusions decreased by 34%, plasma transfusions decreased by 55%, and platelet transfusions decreased by 34%. These decreases have reduced the number of patients exposed to the possibility of transfusion-adverse reactions and related adverse effects. Reported transfusion reactions since 2012 decreased by 51%. By using a more restrictive set of guidelines, the blood utilization committee was able to achieve their goals, which included (1) reducing the high cost associated with blood transfusion, (2) improving patient care, and (3) educating physicians regarding current transfusion protocols and techniques, while concurrently decreasing transfusion-related adverse events. The successful process used at FMH may be useful for other similar health care settings.
- AABB - American Association of Blood Banks
- APTT - activated partial thromboplastin time
- ARC - American Red Cross
- DDAVP - desmopressin
- FMH - Frederick Memorial Hospital
- FRHS - Frederick Regional Health System
- HUS - hemolytic uremic syndrome
- ICU - intensive care unit
- INR - international normalized ratio
- IPF - immature platelet fraction
- JC - The Joint Commission
- RET-He - reticulocyte hemoglobin equivalent
- TTP - thrombotic thrombocytopenic purpura
INTRODUCTION
The Joint Commission (JC) requires the medical institutions it governs to conform to a list of regulations, including the formation of blood utilization guidelines. By establishing guidelines for blood utilization, hospitals and other medical facilities are able to adhere to JC’s regulation for continual improvement of health care for all patients.1 At JC’s overuse summit in 2012, it was noted that red blood cell transfusions were the most commonly performed and the most overused procedure in US hospitals.1 Blood utilization guidelines can help hospitals reduce the number of blood transfusions by educating physicians on new standards that indicate the symptoms and laboratory results that deem it necessary for a patient to receive a blood product transfusion.
Frederick Memorial Hospital (FMH), an affiliate of Frederick Regional Health System (FRHS), is a 240-bed local hospital in Frederick, Maryland. It is a not-for-profit, short-term, acute care facility. FRHS consists of multiple off-site locations that offer a variety of services, including outpatient laboratory and radiology services, home health services, hospice care, oncology care, wellness and fitness support, corporation health services, and a large network of physician offices that offer multiple medical specialties.2 FMH is not a trauma center, but it has been used for trauma situations in which a patient cannot make the trip to a trauma center without being stabilized.
Prior to 2012, the transfusion guidelines at FMH consisted of 5 rules: (1) a patient with normovolemia and symptomatic anemia regardless of their hemoglobin value; (2) acute loss of greater than or equal to 15% of estimated blood volume; (3) acute blood loss with evidence of inadequate oxygen delivery; (4) preoperative hemoglobin less than or equal to 8 g/dL and operative procedure associated with major blood loss; and (5) hemoglobin less than or equal to 8 g/dL in a patient on a long-term transfusion regimen. These guidelines were internal suggestions that physicians could use to determine if a transfusion of any blood product was needed for their patients. Unfortunately, these guidelines were not enforced and, therefore, not always followed by all of the ordering physicians.
In 2012, a premiere report identified that FMH transfused more blood products than their peers. The blood bank supervisor, Lori Park-Dovell, researched the American Association of Blood Banks (AABB) recommendations, attended American Red Cross (ARC) lectures, and called local hospitals to determine recommendations for the FMH physician staff. Subsequently, a blood utilization committee was formed, and they decided to revise their blood utilization guidelines with goals to reduce the high cost of blood transfusions, improve patient care, and educate physicians on newer ideas and/or techniques. The blood utilization committee consisted of the blood bank supervisor, the medical director of the laboratory, pathologist associates, clinical specialist nurses, and several hospital affiliated physicians. The committee communicated with the physicians and used their input to determine the new guidelines. Specific care was taken toward both cardiac and oncology patients because these patient populations are at risk for needing blood products to treat their conditions. The new guidelines were introduced to physicians through education and facilitated with assistance from the laboratory information system software, Meditech, and the blood bank technologists. Physicians were required to fill out transfusion order forms that listed the new guidelines to encourage them to document the reason for the transfusion order. They were required to write down laboratory results, such as hemoglobin and/or hematocrit or platelets counts, to support their desire to transfuse their patients. Progress reports were evaluated monthly, and the blood utilization committee addressed any concerns with the ordering physicians or their direct supervisor.
MATERIALS AND METHODS
New FMH Guidelines 2012
The new guidelines set up by the FMH blood utilization committee took time and research to determine the criteria for blood product transfusions. All blood product transfusion thresholds were derived from data and research provided by the experts at the ARC and AABB. Based on published studies, it was suggested that a restrictive transfusion trigger should be used, and each patient needed to be assessed individually and carefully. For example, a hemoglobin value should be used when considering a patient for a transfusion.3 Although there are limited data available, the lowest threshold tested was a hemoglobin of 7 g/dL. However, patients may still not require a transfusion even if the trigger level of hemoglobin is reached. In addition, physicians need to consider a physical examination and carefully evaluate the patient’s history. If the patient is clinically stable, the physician can refrain from transfusing blood products. For oncology patients, transfusions can be based on symptoms as opposed to only looking at the patient’s test results. Data suggest that gastrointestinal bleed patients should be included in the restrictive transfusion plan. Research has proven that there is an increase in the 45-day survival rate with restrictive therapy. However, transfusion guidelines should not replace clinical judgement. Table 1 shows a comparison of the 2008 and 2012 triggers for blood product transfusions.
Comparison of 2008 and 2012 FMH guidelines
Red Blood Cell Transfusion Recommendations
Red blood cell transfusion guidelines at FMH are based on hemoglobin, hematocrit, and patient symptoms. Any patient who has rapid blood loss with an estimated blood loss of greater than 20% and is not responding to volume resuscitation or with ongoing blood loss do not require specific hemoglobin or hematocrit values to receive red blood cell transfusions. The physician can document the specific reason justifying a red blood cell transfusion for a patient. This case would then be reviewed by the blood utilization committee at their quarterly meeting.
Several trials were studied and reviewed by the ARC and AABB to evaluate the risk bias, allocation concealment, blinding, and incomplete outcome data. The relative risk was also evaluated in each trial to compare the control group with the intervention group. A summary of several clinical trials prepared by the ARC was used to aid in the selection of FMH’s blood utilization guidelines. For example, the transfusion requirements in critical care trial tested 838 ill patients with euvolemia at both hemoglobin levels of 9 g/dL and 7 g/dL.3 Patients were randomly assigned to the restrictive transfusion group and the conservative group. Both groups had similar mortality rates after the 30-day trial.3 Acute Physiology and Chronic Health Evaluation II scores were calculated for each group of patients. The rates were significantly lower in the restrictive transfusion group at 8.7% compared with 16.1% in the conservative transfusion group.3
The transfusion trigger trial for functional outcomes in cardiovascular patients undergoing surgical hip fracture repair (FOCUS) trial researched high-risk patients after they experienced a hip operation. This trial used hemoglobin values of 10 g/dL and 8 g/dL to differentiate between their restrictive and liberal groups of patients.3 Restrictive patients on average received no red blood cell transfusions, whereas the liberal patients on average received 2 units of red blood cells.3 Primary outcome rates of death or being unable to walk across the room without assistance were as follows: the restrictive group was 34.7% and the liberal group was 35.2%.3
For the transfusion requirements in the pediatric intensive care unit (ICU) trial, hemoglobin thresholds of 9.5 g/dL and 7 g/dL were used to determine which group patients were randomly placed in with regard to the restrictive or liberal group.3 Patients in the restrictive group were able to maintain their lower hemoglobin value over the liberal group.
Overall, the studies showed that patients with lower hemoglobin values remain stable without needing a red blood cell transfusion. FMH’s guidelines align with those suggested in the AABB publication. The AABB technical manual clearly states that any patient with a hemoglobin level less than 6 g/dL almost always requires a red blood cell transfusion, and patients with a hemoglobin level greater than 10 g/dL rarely require a red blood cell transfusion.7
Platelet Transfusion Recommendations
Platelet transfusion guidelines are based on the patient’s symptoms and platelet values. Any patient who has been diagnosed with a platelet dysfunction disorder documented by a test result or medication is recommended to receive a platelet transfusion without having a specific platelet count. In 1994, an article published in the journal Transfusion, although not an official recommendation of the AABB, reviewed and suggested transfusion guidelines for several blood products. It recommended that patients should receive a platelet transfusion to “prevent or control bleeding associated with deficiencies in platelet number or function.”5 Patients who are not bleeding, cannot produce platelets, and have a platelet count of less than 10 000/µL were recommended to receive a platelet transfusion.5 A patient with a platelet count of less than 50/µL, who is having an invasive or noninvasive operation, is suggested to receive a platelet transfusion.5 The AABB technical manual discusses the need to not only take into consideration the platelet count, but also several other factors before deciding if a transfusion is necessary. Patients may need to have platelet function and coagulation deficiency testing, and the patient’s history should always be taken into consideration.4 Some actively bleeding patients or those patients having an operation that may involve significant blood loss can benefit from thromboelastography testing. Current thresholds provided by AABB can be found in Table 2. These guidelines are similar to those chosen by the blood utilization committee at FMH, described at the beginning of this section.
Current AABB platelet transfusion guidelines7
Plasma Transfusion Recommendations
Plasma transfusion guidelines are based on both a patient’s symptoms and/or their international normalized ratio (INR) or activated partial thromboplastin time (APTT) results. Patients who need an emergency reversal of warfarin treatment or are diagnosed with thrombotic thrombocytopenic purpura (TTP) or hemolytic uremic syndrome (HUS) are recommended to receive a plasma transfusion without having a specific laboratory value. Patients who have an INR result greater than 1.6 or an APTT greater than 40 are recommended to receive a plasma transfusion if they are also actively bleeding or undergoing an invasive procedure.
There are very limited guideline recommendations from the AABB Technical Manual because sparse research data are available. Massive transfusion scenarios and patients who require warfarin reversal are the main critical reasons for a patient to receive a plasma transfusion.4 Because the research data are very limited, as previously stated, there are currently no guidelines for transfusions in patients who are not in a critical situation. This field needs to be further researched to determine if there are other scenarios that would suggest the need for a plasma transfusion.
Cryoprecipitate Transfusion Recommendations
Cryoprecipitate transfusion guidelines are based on both a patient’s symptoms and their fibrinogen value. Any patient with a fibrinogen value less than 100 mg/dL is recommended to receive a cryoprecipitate transfusion. When desmopressin (DDAVP), Humate-P, or any other comparable factor concentrate is not available, a patient with von Willebrand disease is recommended to receive a cryoprecipitate transfusion. A patient in renal failure with an abnormal clotting time and is either actively bleeding, had an operation, or had an invasive procedure within the last 24 hours is recommended to receive a cryoprecipitate transfusion.
The AABB technical manual states that the fibrinogen level is extremely important to determine if a cryoprecipitate transfusion is needed. It is recommended that a patient maintains a fibrinogen level of at least 100 mg/dL.4 If a patient is consuming fibrinogen because of being in disseminated intravascular coagulation or losing fibrinogen because of a massive hemorrhage, cryoprecipitate product may be required to increase the fibrinogen level in the patient.4 The most important part of transfusing cryoprecipitate is knowing how many units are required to maintain a specific fibrinogen level. Cryoprecipitate is a low-volume–blood product, and a patient may require several units to achieve the desired fibrinogen level.
RESULTS
Red Blood Cells
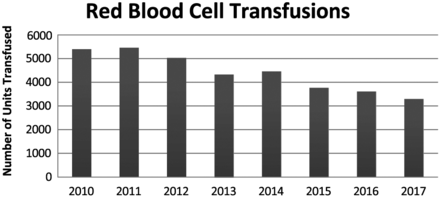
Overall, the number of blood products transfused at FMH has been in decline since the introduction and reinforcement of the 2012 blood utilization guidelines. Yearly totals of red blood cells transfused are shown in Figure 1.
Total number of both irradiated and nonirradiated leuko-reduced red blood cell transfusions at FMH from 2010–2017.
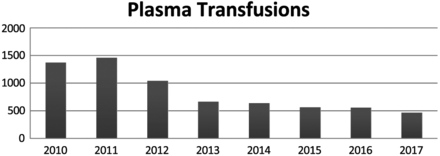
Total number of fresh frozen plasmapheresis transfusions at FMH from 2010–2017.
In 2010, 5401 irradiated and nonirradiated red blood cell products were transfused. In 2013, there was a decrease of red blood cell products transfused (4329 vs 5401). In the year 2017, there were only 3297 red blood cell products transfused. This is a 39% decrease since 2010 and a 24% decreased since 2013.
Plasma
This constant declining trend continues with the plasma transfusions recorded at FMH (see Table 2). In 2012, there were 1041 units of plasma transfused, and, in 2017, there were 465 units of plasma transfused. Therefore, there is a decrease of 55% of plasma units transfused over the course of 5 years. By far, this is the best improvement score across all of the blood products transfused at FMH.
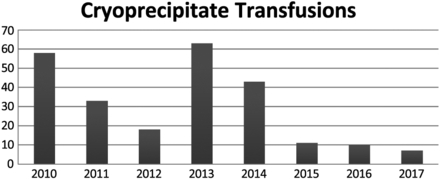
Cryoprecipitate
Cryoprecipitate has always been the lowest volume of products transfused. As seen in Figure 3, the number of cryoprecipitate units has fluctuated through the years because of the types of patients admitted to FMH requiring a cryoprecipitate transfusion. FMH is not a trauma center and, therefore, does not receive patients who may require a massive transfusion. In 2010, 58 units of cryoprecipitate were transfused, and, in 2012, only 18 units were transfused. In 2013, the number of units of cryoprecipitate transfused increased to 63 units. In 2017, only 7 units of cryoprecipitate were transfused.
Total number of cryoprecipitate transfusions that have taken place at FMH from 2010–2017.
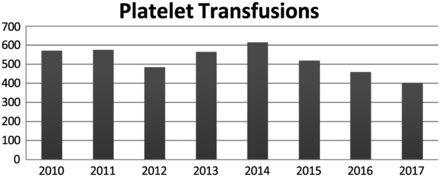
Platelets
Both irradiated and nonirradiated platelets are a constantly transfused product at FMH because of the MD Anderson Cancer Center association with the hospital. The number of platelet products transfused is shown in Figure 4. As with cryoprecipitate products, the number of platelets transfused has fluctuated since 2010. Between 2010 and 2012, there was a 17% decrease in the number of platelet products transfused. However, in 2014, there was a slight increase in the total number of platelet units transfused. This increase could be caused by an increase in the oncology population being treated at our cancer treatment center requiring platelet transfusions. From 2014–2017, there has been a 34% decrease in the number transfused.
Total number of both irradiated and nonirradiated platelet apheresis transfusions at FMH from 2010–2017.
Transfusion Reactions
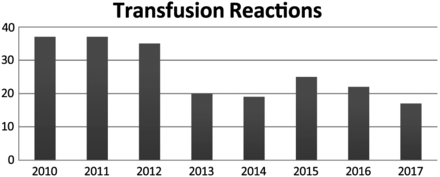
One of the biggest risks for a patient when receiving any kind of blood product is the exposure to possible reactions to the transfusion itself, including illness, allergies, and even death. Over the last 7 years, the number of transfusion reactions has drastically decreased, possibly because of the decrease in the number of products transfused. Values for the number of transfusion reactions are shown in Figure 5. Since 2012, the number of transfusion reactions has decreased by 51%. This is a significant improvement in the number of reactions. To illustrate the breakdown by categories of infusion reactions, in 2017, 3 of the 17 recorded transfusion reactions were determined to be an allergic or anaphylactic reaction to the product. 10 of the recorded transfusion reactions were because of febrile, nonhemolytic reactions to the product, and 4 of them were determined to be another reason other than those typically reported. The 4 outliers may be incorrectly called transfusion reactions.
Total number of recorded transfusion reactions that occurred at FMH from 2010–2017.
DISCUSSION
As seen in the studies provided by the ARC, patients tend to have better outcomes when treated with restrictive blood utilization guidelines.3 This is because fewer patients are being exposed to potential adverse effects associated with blood product transfusions. Instead of transfusions, patients are being treated with medication or naturally by allowing the patient’s blood production system to improve their status.
Based on these findings, FMH decided to adjust their blood utilization guidelines in 2012 with the goals of reducing the cost of blood transfusions, improving patient care, and educating physicians to use the new guidelines appropriately as part of their clinical tools. Since 2012, the blood bank at FMH has been able to reduce the amount of inventoried red blood cells kept at the hospital. This outcome was observed in 2 similar studies that showed a reduction in red blood cell utilization. For example, a study at Stanford Hospital and Clinics used real-time clinical decision support to assist physicians when placing orders for blood product transfusions. At the time the process was initiated in 2009 until 2012, the percentage of patients transfused with red blood cells for patients with a hemoglobin value greater than 8 g/dL decreased from 66% to less than 30%.6 Overall blood components also decreased during this timeframe, resulting in a cost saving of greater than 1.6 million dollars.6 Another study at the John Hopkins Hospital System established a patient blood management program that initiated a campaign for promoting single-unit red blood cell transfusion. Whenever a patient’s hemoglobin value was greater than 7 g/dL, a “pop-up” box would appear when the physician ordered a red blood cell transfusion.7 The message encouraged a transfusion protocol for using a single unit of red blood cells and rechecking the patient’s hemoglobin and hematocrit values before transfusing another red blood cell unit.7 This program decreased the red blood cell utilization by 27.2% across the 3 hospitals within the Johns Hopkins Hospital System.7 Both the Stanford and Johns Hopkins studies proved that there are several methods that hospitals can employ to encourage physicians to conform to a more strict blood utilization process. Like the results seen in our study, these data further demonstrate that blood utilization programs are effective in reducing blood product utilization.
For the new set of blood utilization guidelines to work effectively, it was essential to educate and enforce on FMH physicians and order nursing staff to follow them. Without this step, the entire project would not have been as successful. Also, monthly reports are evaluated by the blood bank supervisor to review all blood production transfusions and the laboratory test triggers. It is up to the discretion of the blood bank supervisor to submit any information about patients whose results and transfusion history do not follow the current blood utilization guidelines to the blood utilization committee. The pathologists on the blood utilization committee evaluate the reviewed patient information quarterly to determine if the transfusion was necessary. The committee invites the physician who ordered the transfusion to participate in the review. From the discussions during the review process, the committee determines what steps need to be taken to avoid unnecessary transfusions in the future.
Future Directions
Recently, FMH implemented a new instrument for processing complete blood counts, the Sysmex XN-3000 (Kobe, Japan). In addition to the continued use of the blood utilization guidelines, this machine offers 2 new tests that could reduce red blood cell and platelet transfusions. The 2 tests are the immature platelet fraction (IPF) and reticulocyte hemoglobin equivalent (RET-He). IPF is a measurement aimed to evaluate thrombopoiesis in patients. As the bone marrow produces platelets, the IPF level in a patient increases as well.8 Patients with a low platelet count (<50 x 109/L) will automatically have an IPF result generated. This laboratory test does not replace the platelet count as the deciding factor in assessing the need for a platelet transfusion but can be helpful in determining if the patient has a production or destruction problem. Sysmex sponsored a study for predicting thrombopoietic recovery in patients who received autologous stem cell transplantation. It is common for a patient who received a stem cell transplant to also receive a prophylactic platelet transfusion regardless of the patient’s platelet count.9 The study determined that a cutoff value of 5.3% IPF would predict thrombopoietic recovery within 2 days of a stem cell transplant.9 By allowing a patient’s own body to recover and produce platelets, patients are less likely to require a platelet transfusion and be exposed to the possible adverse effects associated with a transfusion.
RET-He, which is a test that measures the hemoglobin content in circulating reticulocytes, is a useful laboratory tool to monitor and diagnose iron deficiency anemia.10A RET-He result would allow a physician to first determine if iron deficiency is the cause of their patient’s anemia and have the opportunity to prescribe iron vitamin supplements before ordering a red blood cell transfusion. RET-He testing is performed with every reticulocyte count ordered in FMH. A study in the ICU of Hospital Sant Joan de Déu in Spain used RET-He to examine patients functioning with iron deficiency and the requirement for blood transfusions while admitted to the ICU. The findings of this study confirmed that a lower RET-He value required higher transfusion requirements.11 Consequently, the IPF and RET-He tests will offer physicians more tools to help diagnose and determine the right treatment plans for their patients and have the potential to enhance the outcomes of the current blood utilization guidelines. To evaluate the effectiveness of the IPF and RET-He tests in the future, the blood utilization committee will compare transfusions and the ordering of these 2 tests.
- Received March 14, 2019.
- Accepted September 16, 2019.
American Society for Clinical Laboratory Science