This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Virginia Commonwealth University
- Virginia Commonwealth University
- Virginia Commonwealth University
- Virginia Commonwealth University
- Virginia Commonwealth University
- Virginia Commonwealth University
- Address for Correspondence: Thomas Corey Davis
, Virginia Commonwealth University, tcdavis{at}vcu.edu
ABSTRACT
Infection control concerns abound in the surgical anesthesia workstation, placing patients and providers at significant, documented risk because of many factors, including provider hand hygiene lapses, equipment design and complexity, and challenging disinfection. A trial was performed to mitigate cross-contamination involving 30 general anesthesia surgical operations matched 1:1 as control (no intervention) or intervention group (condom-like barriers to 4 anesthesia workstation components that are frequently touched and contaminated and very difficult to disinfect). Wraps were removed at the end of the operation and then replaced with fresh ones before the start of the subsequent operation. Baseline culture samples were obtained prior to the first surgical operation of the day in each room and then performed on operations that followed in each room over a 3-day period. Baseline colony-forming unit density was equivalent in both conditions with total density significantly lower in the covered/wrapped (mean rank = 5.81) vs uncovered condition (mean rank = 11.19) at P < 0.01, r = −0.64. Bacterial species diversity was markedly decreased in the covered condition. The covered condition served as a barrier to contamination of apparatus elements, preventing downstream patient exposure and mitigating between-procedure disinfection need. Intervention group providers were debriefed, finding only rare, addressable concerns. This research further validates the need for routine, periodic culturing of anesthetic apparatus to reveal lapses in provider behaviors and disinfection practices.
- ADM - anesthesia delivery machine
- APL - adjustable pressure-limiting
- AW - anesthesia workstation
- CFU - colony-forming unit
- EMR - electronic medical record
- HAI - health care–associated infection
- IV - intravenous
- IRB - institutional review board
- OR - operating room
- PYR - pyrrolidonyl arylamidase
- SBA - sheep blood agar
- VCUMC - Virginia Commonwealth University Medical Center
INTRODUCTION
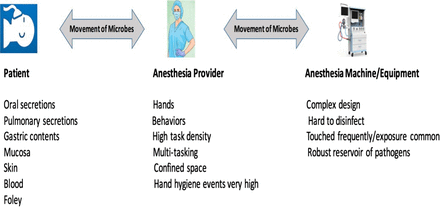
There are significant infection control concerns in both provider behavior and equipment domains present in the surgical anesthesia workstation (AW), placing patients and their providers at substantial risk.1⇓⇓⇓-5 There is a clinically significant potential for microbial cross-contamination from 1 patient to subsequent patients scheduled in the same operating room (OR) because of lapses in hand hygiene, procedural task density, equipment design complexity, and difficulty in disinfecting the apparatus between patients (Figure 1).
Vectors of microbial contamination in the AW.
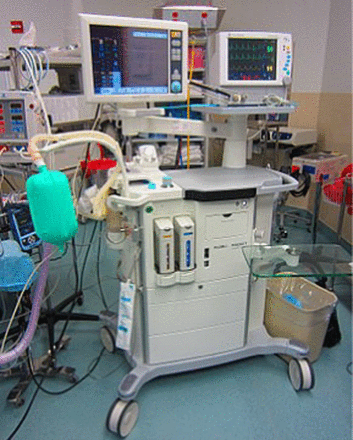
During the anesthetic treatment of the surgical patient, the AW represents a challenging environment where it is nearly impossible to consistently perform optimal asepsis when rendering care (Figure 2). Work from both our laboratory and clinical studies,6⇓-8 as well as from others,1⇓⇓-4,9 demonstrates quantifiable risks of microbial cross-contamination in the AW that must be addressed. Health care–associated infections (HAIs) are a significant national health concern carrying increased patient morbidity, mortality, and financial cost.10,11
A typical anesthesia machine with architectural/design complexity, making cleaning and disinfection between surgical procedures very challenging.
Recent multidisciplinary expert guidance statements endorsed by the American Operating Room Nursing Association, the American Society of Anesthesiologists, the American Association of Nurse Anesthesiology, and the American Association of Anesthesia Assistants detail the grave risks of microbial iatrogenesis in the AW.5 Several of these recommendations relate to the need for ongoing patient safety research regarding between-procedure decontamination of the anesthesia delivery machine (ADM) as well as the potential role of disposable covers/wraps to prevent microbial contamination. This authoritative guidance noted a deficiency of, and need for, evidence-based research targeting these issues.
A clinical pragmatic trial was designed to assess a novel approach to mitigate the risk of cross-contamination in the AW. The following hypothesis was tested: Strategic barriers placed on the ADM during general anesthesia administration will decrease density and diversity of bacterial contamination over the course of sequential patients exposed to the same equipment compared with machines without the barriers.
MATERIALS AND METHODS
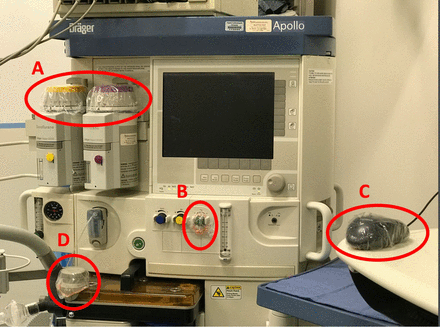
With institutional review board (IRB) approval from Virginia Commonwealth University Medical Center (VCUMC), 30 diverse surgical operations requiring general anesthesia care over a 3-day period were matched 1:1 as the control group (N = 15, no intervention, the uncovered condition) or the intervention group (N = 15, condom-like barriers to anesthesia machine hot spots [defined as AW elements frequently touched and difficult to disinfect, revealed in our previous work], the covered condition). These included the electronic medical record (EMR) computer control mouse, the breathing circuit pressure control (or adjustable pressure-limiting [APL]) valve, the oxygen flowmeter control, and the anesthetic agent vaporizer dials (Figure 3). This prospective, pragmatic trial involving a convenience sample of surgical patients was conducted in the main operating suites of VCUMC.
Location of condom-like protective covers: (A) vaporizer control dials, (B) oxygen flowmeter control, (C) electrical medical record control mouse, and (D) breathing circuit pressure control valve (APL valve).
A supply of elasticized condom-like covers was obtained in 3 sizes (small, medium, and large). In a prestudy test, it was established that these were nonpermeable to bacteria congruent with the full anesthesia machine wrap from the previous study.8
Preliminary Study: Assessing Wrap Integrity
A preliminary study was performed to test the ability of the plastic covers to prevent bacterial contamination of the ADM. A sterile condom-like cover was applied to the vaporizer dial of an ADM. The dial was scrubbed clean with ethanol prior to the application to ensure that prior contamination did not affect the results. ESwabs containing Amies media (COPAN Diagnostics Inc, Murrieta, CA) were then used to collect samples from the plastic cover (both sides) and the anesthesia machine prior to the inoculation with Staphylococcus epidermidis (ATCC 12228). The sterile plastic cover was applied over the cleaned anesthesia machine dial. Approximately 5 colonies of S epidermidis (ATCC 12228) were removed from a blood agar plate with a sterile swab and inoculated and spread onto the outside of the cleaned plastic cover. ESwabs were used to swab the outside of the plastic cover, inside of the plastic cover, and covered dial to determine if the plastic cover prevented the contamination of the ADM.
The collected swabs were taken back to the laboratory and vortexed for 2 minutes. Immediately following the vortexing, 100 µL of Amies media was removed from the ESwab tube and plated directly to a sheep blood agar (SBA) plate (Thermo Fisher Scientific, Waltham, MA). The inoculum was spread evenly over the plate using a cell spreader, and the plates were incubated for 48 hours at 35 °C to allow for bacterial growth. No bacterial growth was observed on the 3 plates prepared using samples collected prior to the addition bacteria, indicating that the ADM (area covered) and plastic cover were free of detectable bacterial contamination prior to the inoculation with S epidermidis. The only plate to demonstrate bacterial contamination from the samples collected after the addition of bacteria was the plate inoculated with the ESwab from the outside of the plastic cover. Plates prepared using samples from the inside of the cover and the knob were both free of detectable bacterial contamination, indicating that the covers prevent the contamination of the dial (Figure 4). The organism grown was then identified as S epidermidis using Gram staining, catalase testing, coagulase testing, pyrrolidonyl arylamidase (PYR) testing, and novobiocin susceptibility testing.
Preliminary study to assess wrap barrier effectiveness. Growth on SBA after 48-hour incubation (A) outside of plastic cover after inoculation with S epidermis, (B) inside of plastic cover after inoculation with S epidermis, and (C) knob covered after inoculation with S epidermis.
Conduct of the Primary Study
Ethical Considerations
With IRB assent, the study was conducted in a manner that did not deviate from the routine surgical and anesthetic treatment of the enrolled patients. Enrollment of patients in the intervention group was approved by both surgical and anesthesia team members as well as the director of OR services, all of whom were aware of the study goals and methodology. Confidentiality of patient information was assured as a given case became a randomly selected rubric, and no identifying patient information of any kind was recorded.
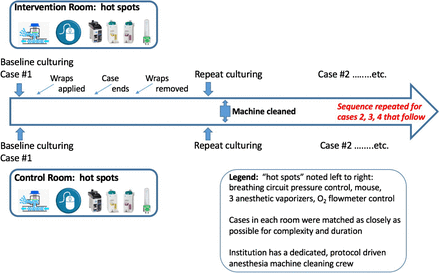
Culture samples were obtained from the 4 hot spots prior to the first surgical operation of the day, after the provider had completed their routine anesthesia machine checkout procedure to ensure full functionality. This was done for each of the 2 matched rooms for each of the 3 days of the study. Rooms selected on each day of the study were matched based on procedure similarity, with 1 room randomly chosen as the intervention room and the other as the control room. To prevent contamination in the intervention room, the study team used hand sanitizer and donned gloves prior to placing the covers.
Immediately following each operation, after both patient and provider exited the OR for transfer to the postanesthesia care area or intensive care unit, repeated cultures were obtained from each of the hot spots after careful removal of the covering (employing the same hand sanitizing/gloving protocol as before).
The researchers interfered with neither the environmental services staff who routinely cleaned the AW between operations, nor the anesthesia technician staff who prepared the AW for the next operation. The sequence was repeated in both the intervention OR and the control OR over the course of 3 consecutive days (Figure 5).
Chronology of culturing in the intervention and control room cases.
Bacterial Culture Analysis
Samples were collected for bacterial culture analysis using ESwabs (COPAN Diagnostics Inc, Murrieta, CA) from 4 designated target surfaces on the anesthesia machine in both the intervention and control rooms. Prior to use, each ESwab was coated with tween, a nonionic surfactant, and excess solution was removed by pressing the tip of the swab against the wall of the tube. The target surface was swabbed using a rotating and twisting motion and immediately placed in 1 mL of liquid Amies media. Once in the laboratory, each ESwab was vortexed for 2 minutes, and 100 µL from each respective sample was removed to inoculate SBA and MacConkey agar (COPAN Diagnostics Inc, Murrieta, CA). Inoculated agar plates were incubated at 35 °C for 48 hours to observe bacterial growth. Colonies observed were examined based on colony morphology, and colony-forming units (CFUs) were recorded. Colonies were then subcultured on SBA for additional isolation and identification.
Identification was based on colony morphology, Gram stain, and rapid spot tests, such as catalase, coagulase, Staphaurex Plus latex agglutination, PYR, Remel Microdase discs, indole, and oxidase (all from Thermo Fisher Scientific, Waltham, MA).
Postprocedural Debriefing
At the end of each operation in the intervention group, the 15 providers were given the opportunity to comment on the covers in a deliberately unstructured manner (to minimize bias) to assess any concerns or perceptions that they wanted to voice with regard to the covers.
Data Analysis
Mann-Whitney tests were used to examine the differences in total CFU density between the covered and uncovered group conditions overall, between each procedure, and between the 4 locations or hot spots and to establish baseline equivalency. Mann-Whitney tests are the nonparametric equivalent of the independent samples t test and are appropriate to use when testing for differences across 2 independent conditions. Effect size (r) was calculated and reported for each significant result. All data were analyzed using Statistical Product Service Solutions Statistics 26 (IBM).
RESULTS
Quantitative Component: Bacterial Contamination
At the start of each day, baseline CFU density was equivalent in both conditions. Total CFU density was significantly lower in the covered (mean rank = 5.81) vs uncovered condition (mean rank = 11.19) at P < 0.01, r = −0.64. This demonstrated a powerful protective effect of the wrapping material.
The ADMs from the intervention group demonstrated an overall decrease in CFUs (Table 1). The highest CFU count for both groups was seen from samples collected from the oxygen flow valve. The control group had 1 case with 39 500 CFU/mL on this hot spot vs the intervention group, which demonstrated a case with 660 CFU/mL. Additionally, there were 5 cases (5/16, 31.25%) that demonstrated no bacterial contamination on any of the hot spots cultured in the intervention group. This was not seen in the rooms that did not have covers over the hot spots. All samples collected from these cases (15/15) demonstrated bacterial contamination on at least 1 area tested.
CFU range for control group vs intervention group
Laboratory results demonstrated that these isolates were gram-positive cocci in clusters, catalase positive, and coagulase positive. Isolate identification was confirmed to be Staphylococcus aureus with a positive result using the Staphaurex Plus latex agglutination test (Thermo Fisher Scientific, Waltham, MA).
There was a decrease in bacterial diversity for the covered oxygen flow valves, vaporizer dials, and computer mouse (Table 2). The oxygen flow knobs were the only hot spot contaminated with S aureus. This potential pathogen was isolated from 2 patients, both from the control group.
List of bacteria cultured from the anesthesia machine
Qualitative Component: Provider Comments
Postoperation provider debriefings noted rare concerns regarding device performance that require follow-up modification. These primarily involved the device slipping off AW components (3/15 providers), failed traction of the device on the associated component (2/15 providers), and impeding smooth movement of the EMR mouse (3/15 providers). None of the concerns were considered insurmountable by the reporting provider, and concerns are being addressed with attention to fit and texture of device fabric.
DISCUSSION
The Centers for Disease Control and Prevention published a white paper urging health care providers and institutional leadership to address the following short- and long-term goals: (1) preventing iatrogenic infection for those having surgical interventions, (2) preventing the spread of bacterial organisms, and (3) avoiding misuse of antibiotics.12 With this mandate, as well as that of the recently published guidelines5 urging research directed specifically at the AW, the current pragmatic trial was taken on.
The perioperative milieu is a reservoir of bacterial (and, for that matter, viral and fungal) organisms that are pathogenic to humans. In recent work, it appears that 50% of surgical site infections are linked to bacterial pathogens that were in the OR where the patient’s procedure was performed.4 There is compelling and methodologically sound research that demonstrates that the endemic nature of institutional HAI is, in part, because of the inherent complexity and strength of biofilm formation and its resistance to mechanical and chemical assault, making aggressive action in hand hygiene, equipment cleaning/disinfection, and antibiotic stewardess critical areas to target.13,14
Anesthesia care routinely involves invasive procedures, such as intravenous (IV) line placement, endotracheal intubation, and the use of needles to access nerves and the subarachnoid space, the latter to create what is known as regional anesthesia. These all bypass the usual first-line defense mechanisms of the body. Open-lumen IV access stopcocks are used in direct and continuous continuity with the patient’s circulating blood and are known to be contaminated with pathogenic organisms in about one-third of surgical procedures.3,5,15 Using sophisticated techniques (e.g., cell genome identification, pulsed-field gel electrophoresis), organisms cultured at the open-lumen stopcock are directly linked to postoperative infection development in addition to being associated with increased postoperative death.3,5,15
The presence of pathogens of any kind (bacterial, viral, and fungal) on high-touch areas (such as those in the present study) and the ease of their transfer to a patient’s IV access ports and to their airway during even routine care merits study and innovation to establish strategies that may prove beneficial in reducing the risk of AW-related infectious iatrogenesis. That providers wear disposable gloves is an essential component of patient care; gloving or wrapping the high-touch, difficult-to-clean ADM components seems rational on a foundational level.
The few deficiencies and concerns noted in the qualitative component of the study were largely related to the wraps slipping off. This occurred on 2 occasions with the oxygen control, likely explaining contamination in the intervention group. There were no patient safety concerns raised (e.g., hindrance of care) by the providers in the intervention group. These deficiencies are currently being addressed with attention to fit and texture of device fabric.
With respect to disinfection of equipment prior to subsequent patients coming into contact with it, the covered condition (i.e., machines with wrap barriers in place) not only served as a barrier to contamination of apparatus hot spots but served as a preventive measure to subsequent downstream (next-patient) exposure. This was a function of cover being removed at each procedure end, thus mitigating the need for between-procedure disinfection.
Although the study was conducted in a single, large metropolitan facility in the United States, the implications are significant both domestically and internationally where infectious disease transmission remains a significant public health issue. The COVID-19 crisis is but 1 condition that exemplifies the very real concerns with doing everything possible to mitigate the risk of pathogenic transmission in the health care setting.
Limitations
The study was not randomized and was performed at a single center. Although providers were not informed of the study purpose, and cultures were taken when they were not in the room, it is likely that a Hawthorne effect may have been present over the 3-day period. Furthermore, covers were not custom designed to the ADM components, and thus optimization of their effectiveness was not likely. Additionally, only bacterial contamination was studied; fungal (e.g., Candida auris) and viral (e.g., hepatitis C virus, COVID-19) pathogens were not assayed.
CONCLUSION
Previous work demonstrated that use of a full apparatus barrier conveyed significant benefit to the patient in mitigating the risk of cross-contamination yet may meet resistance by providers because of access constraints. In this proof-of-concept, clinical pragmatic trial, it was demonstrated that strategic barrier applications to high-touch, universally contaminated niches on the ADM conveyed patient safety benefits by theoretically reducing the risk of AW-acquired infection and add to the call for evidence noted in the recent authoritative guidance document. This research further validates the need for routine, periodic culturing of anesthetic apparatus to reveal lapses in provider behaviors and disinfection practices.
Future research should include a larger sample size, multi-institutional representation, and an even broader patient population, including obstetrical and pediatric patients. Likewise, investigating the use of novel techniques, such as that described in the current study targeting fungal and viral pathogens, is warranted.
- Received January 13, 2020.
- Revision received March 2, 2020.
- Accepted March 9, 2020.
American Society for Clinical Laboratory Science