This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- University of Texas Medical Branch
- University of Texas Medical Branch
- Hamilton General Hospital
- Hamilton General Hospital
- Address for Correspondence: Sanam Koirala
, Hamilton General Hospital, sanambdrkoirala{at}gmail.com
ABSTRACT
BACKGROUND: Warfarin is indicated for the prevention and treatment of venous thrombosis and thromboembolic complications associated with atrial fibrillation. The delivery of high-quality healthcare in a rural hospital requires the same, if not higher, focus on managing patients’ international normalized ratio (INR) within the therapeutic range. Options for warfarin management include anticoagulation clinics, in-home self-testing, pharmacist-led management, and physician-led management. However, rural hospitals are usually unable to afford specialized anticoagulation clinics to monitor patients receiving warfarin therapy. The purpose of this study is to optimize and examine the efficacy of warfarin therapy management in a rural hospital by utilizing the resources available within the hospital through the use of a diagnostic management team (DMT).
DESIGN: In order to evaluate the efficacy of DMT for warfarin management in a rural hospital (Hamilton General Hospital, Hamilton, TX), we conducted a retrospective chart review to analyze the time in therapeutic range (TTR) for the target and expanded therapeutic ranges (±0.2 and ±0.3 INR units), average percentage of INR values in the target and expanded therapeutic ranges, and percentage of INR <1.5 and INR >4.5. These outcomes were compared before and after DMT implementation.
RESULTS: A total of 50 patients (48% male and 52% female) underwent 205 INR measurements before DMT implementation and 247 INR measurements after DMT. The most common indication for warfarin was atrial fibrillation, followed by DVT. TTR for the target range increased from 52% pre-DMT to 64% post-DMT. TTR for the expanded therapeutic range (±0.2 INR units) increased from 64% pre-DMT to 77% post-DMT. Similarly, TTR for the expanded therapeutic range (±0.3 INR units) increased from 69% pre-DMT to 81% post-DMT. The average percentage of therapeutic INRs was 62% pre-DMT and 74% post-DMT (P < .05) for the target range, 72% pre-DMT and 86% post-DMT (P < .05) for the expanded therapeutic range (±0.2 INR units), and 76% pre-DMT and 86% post-DMT (P < .05) for the expanded therapeutic range (±0.3 INR units). The percentage of INR <1.5 decreased by 6.9% and INR >4.5 decreased by 1.6% post-DMT.
CONCLUSION: Incorporating a comprehensive approach for optimizing warfarin therapy through the use of DMT and utilizing the resources available in a rural hospital improved TTR and the percentage of INRs in the therapeutic range for both target and expanded therapeutic ranges and decreased bleeding and clotting episodes as well as warfarin-related documentation. DMT may be an economically attractive alternative platform to prevent bleeding and clotting and improve treatment monitoring for patients on warfarin therapy.
- A-fib - atrial fibrillation
- DCLS - doctorate in clinical laboratory science
- DMT - diagnostic management team
- DVT - deep vein thrombosis
- ER - emergency room
- INR - international normalized ratio
- LIS - laboratory information system
- NAM - National Academy of Medicine
- PE - pulmonary embolism
- PT - prothrombin time
- TTR - time in therapeutic range
- diagnostic management team (DMT)
- Warfarin therapy
- time in therapeutic range, pharmacogenetics
- CYP2C9
- VKORC1
INTRODUCTION
Warfarin is indicated for the prevention and treatment of venous thrombosis and thromboembolic complications associated with atrial fibrillation (A-fib).1⇓⇓-4 It is also used to prevent recurrent deep vein thrombosis (DVT) and pulmonary embolism (PE). Warfarin inhibits the synthesis of clotting factors II, VII, IX, and X, along with the naturally occurring endogenous coagulants protein C and protein S.1,2,5 The earliest changes in international normalized ratio (INR) are typically seen in the first 24–36 hours after administration of a warfarin dose.2,6,7 However, full antithrombotic effects of warfarin do not occur until approximately the fifth or later day of therapy, and frequent monitoring is required for maintaining INR within the therapeutic range.1,6
One of the major risks of warfarin therapy is bleeding, which correlates well with INR values. The effectiveness and safety of warfarin depend on maintaining the INR within the therapeutic range, as shown in Table 1.1,8 Failure to maintain INR within the therapeutic range is often associated with increased risk of hemorrhage or thromboembolism, depending on the direction of INR deviation.2,9 According to one meta-analysis, warfarin is associated with a 0.6% annual risk of bleeding-related death, 3% annual risk of a major bleeding event, and 9.6% annual risk of major or minor bleeding events, with the highest risk occurring around the start of therapy.10
Patient assessment questions incorporated within the diagnostic management team workflow
Warfarin is typically managed by a primary care physician, pharmacist, and nurses in an anticoagulation clinic in many hospitals in the United States. This system requires appointments or telephone calls to discuss INR values and dose adjustments. However, anticoagulation clinics are primarily available in large tertiary care centers, not in small rural hospitals. Similarly, a meta-analysis showed that traditional family physician–led anticoagulation monitoring is not effective for keeping patients within the target INR range.3 Similarly, there are gaps in understanding how health professionals can most effectively train and support patients who are monitoring their INR with home self-testing.11 There are also no data to support the safety and reliability of home self-testing of INR, indicating the need for great caution in offering this type of monitoring to patients.2 Several previous studies suggested that coordinated care and a systematic approach in anticoagulation management improve outcomes, reduce adverse events, and improve patient satisfaction.12 It has been documented that improving patient satisfaction with the therapeutic regime is important for the care of patients with chronic illness who are receiving warfarin.13 The need for regular blood testing, limitations of lifestyle (including restrictions on diet and activities), and fear of bleeding often result in reduced patient satisfaction and quality of life in patients receiving warfarin therapy. Thus, there is a need to improve diagnostic processes in the management of patients receiving this medication.
Healthcare providers in many countries are unaware that poor clinical outcomes could be prevented if diagnostic processes are improved.14,15 According to Singh and Sittig,16 “safer diagnostic processes include five dimensions: the patient provider encounter and initial diagnostic assessment, diagnostic test performance and interpretation, follow up and tracking of test results and patient related factors.” Similarly, a National Academy of Medicine report focuses on the concept that the quality of diagnosis will be highly dependent on an effective diagnostic team.17 Therefore, implementing a diagnostic management team (DMT) is another systematic and coordinated approach to managing warfarin in a rural hospital without an anticoagulation clinic, which requires the same, if not higher, focus on managing patients receiving warfarin as urban and suburban areas. A DMT is a collaborative effort among medical experts centered around a particular diagnostic discipline, with the goal of enhancing diagnostic accuracy and avoiding unnecessary, costly, and inappropriate diagnostic testing.17 A DMT may also include health professionals from associated disciplines, such as primary care, radiology, nursing, laboratory staff, and biomedical informatics, who can add value to the diagnostic accuracy.17 DMTs typically meet on a regular schedule, provide patient-specific diagnostic interpretation and recommendations in real time, document and deliver reports in the medical record before or during the time when treatment decisions are made, and focus on the correct selection of laboratory tests.17,18 The proposal for optimizing warfarin therapy through the use of a DMT is one of the strategies to improve warfarin therapy in rural hospitals lacking resources for managing patient receiving warfarin therapy. DMTs can play a critical role in providing expert recommendations and interpretations regarding therapeutic INR doses for individual patients, which are reviewed with the physician and other DMT members in real time, followed by communication of the recommended dose to the patient on the same day before the patient takes their daily warfarin dose.
METHODS
Hypothesis
The hypothesis of this study was that a DMT at a rural hospital directed by an individual with a doctorate in clinical laboratory science (DCLS) will have positive outcomes for patients and the institution in terms of time in therapeutic range (TTR) for the target therapeutic INR range and expanded therapeutic INR ranges as well as the average percentage of therapeutic INRs in the target and expanded therapeutic ranges and reduce the percentage of INR <1.5 and INR >4.5.
Purpose of the Study
The purpose of the current study was to optimize and examine the efficacy of warfarin management in a rural hospital through the use of a DMT.
Research Questions
The research questions for this study were as follows:
1. Does a DMT implemented and led by a DCLS at a rural hospital lead to positive outcomes for patients and the institution in terms of TTR for target and expanded therapeutic ranges?
2. Does a DMT implemented and led by a DCLS at a rural hospital increase the average percentage of therapeutic INRs in the target range and expanded therapeutic INR ranges?
3. Does a DMT implemented and led by a DCLS reduce the percentage of INRs outside of therapeutic ranges (INR <1.5 and INR >4.5) before and after the implementation of DMT?
4. Does a DMT implemented and led by a DCLS increase the number of therapeutic INR within target and expanded therapeutic ranges before and after the implementation of DMT?
Research Design
The study used both a quasi-experimental design. The quasi-experimental design included evaluating TTR for the target and expanded therapeutic ranges (±0.2 and ±0.3 INR units) and the average percentage of therapeutic INRs in the target and expanded therapeutic ranges (±0.2 and ±0.3 INR units) in patients receiving warfarin at a rural hospital. These data were collected through retrospective chart review both before and after DMT implementation.
Research Setting
The rural hospital is privately owned and located in Hamilton, a small town in central Texas with a population of approximately 3000. The population is mostly White (85%), with 10.8% Hispanic or Latino and 2.3% other races.19 The hospital has 42 beds and 3 clinics, which are located in Hico, Goldhwaite, and Hamilton, Texas. About 40% of the patients at the hospital are insured through Medicare. The hospital performs a number of outpatient services and receives referrals from surrounding areas, including Waco and Temple, Texas. Warfarin was managed by the primary care physician in all 3 clinics.
Participant Recruitment and Selection (Sample)
The study population included 50 adult patients, both male and female, who received warfarin for various indications, including A-fib, DVT, PE, DVT/PE, A-fib/DVT, and aortic valve replacement.
Instrumentation
The Stago Compact analyzer was used to analyze all samples for prothrombin time (PT)/INR. The laboratory information system (LIS) was Medhost, and the EMR system was eclinical works. In order to ensure reliable and valid results from the analyzer, quality control of the instrument was performed every 8 hours, and samples were checked for preanalytic errors prior to testing each sample.
Research Procedure
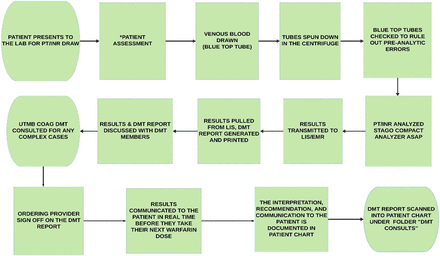
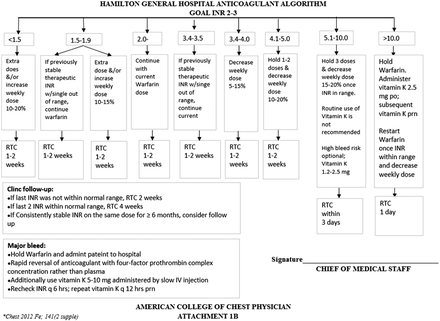
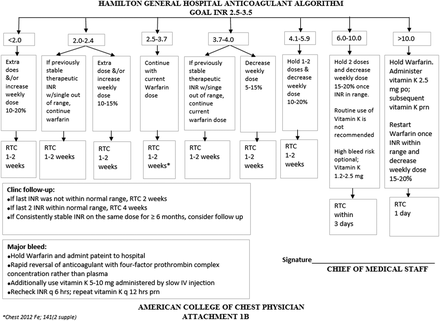
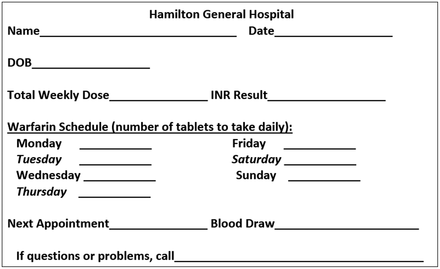
We instituted a DMT on July 17, 2018, which was led by DCLS who was working as a laboratory director at the rural hospital. The DMT workflow at the rural hospital is shown in Figure 1. The DMT for optimizing management of patients receiving warfarin therapy consisted of 6 primary care physicians, 1 physician assistant, 4 nurses, 1 laboratory director, 1 phlebotomist, and 1 laboratory technologist, who met on a regular basis. Two laboratory staff and 4 nurses were trained regarding the DMT workflow on June 15, 2018 and overseen by the laboratory director. The trained laboratory staff obtained answers to the patient assessment questions shown in Table 1, after which they obtained a blood sample in a blue top tube. Each tube was checked to rule out preanalytic errors, such as a small sample, hemolysis, or mislabeling, which could contribute to false results. The PT/INR was determined as soon as possible in the STAGO compact analyzer, and the results were finalized in the LIS. The results were then retrieved from the LIS, and the DMT report was generated by the DCLS laboratory director. This report included the patient’s history, diagnosis, diagnostic test results, interpretation, and recommendations, as shown in Figure 2. For each dosing strategy, an algorithm recommended by the American College of Chest Physicians guidelines (shown in Figure 3 and Figure 4), along with consultation from the DMT members, was used as a guide for warfarin dose adjustment.20 The warfarin dose instruction card shown in Figure 5 was also utilized and given directly to the patient; this was especially important for elderly patients, who tended to be less compliant with warfarin therapy.21 The University of Texas Medical Branch coagulation DMT was consulted for complex cases requiring expert recommendations. The DMT report was then discussed with the DMT members and the ordering provider. The ordering provider signed the report to acknowledge that it was received and added other recommendations to the report that were pertinent to the patient, if necessary. The nurse then communicated the recommendations to the patient on the same day the blood was obtained and documented the recommendations and telephone encounters in the patient’s EMR. The DMT report (Figure 3) was then scanned into the patient’s chart under the folder “DMT consults.”
Warfarin management workflow at the Hamilton General Hospital. DMT, diagnostic management team; EMR, electronic medical record; INR, international normalized ratio; LIS, laboratory information system; PT, prothrombin time; UTMB COAG DMT, University of Texas Medical Branch anticoagulation DMT.
The diagnostic management team report for warfarin management.
Algorithm for the management of patients with an INR goal of 2–3. INR, international normalized ratio; po, orally; prn, as needed; RTC, return to clinic. Retrieved from https://journal.chestnet.org/article/S0012-3692(12)60122-6/fulltext.
Algorithm for the management of patients with an INR goal of 2.5–3.5. INR, international normalized ratio; po, orally; prn, as needed; RTC, return to clinic. Retrieved from https://journal.chestnet.org/article/S0012-3692(12)60122-6/fulltext.
Warfarin dose instruction card. DOB, date of birth; INR, international normalized ratio. Adapted from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6025075/.
Inclusion and Exclusion Criteria
All patients receiving warfarin from all 3 clinics of the hospital were included in the study. The study included only adult patients over the age of 18 years who were diagnosed with an indication listed in Table 2 (A-fib, DVT, PE, DVT/PE, A-fib/DVT, and aortic valve replacement) and who required warfarin therapy. The project did not include pregnant women, children, cognitively impaired people, or prisoners. PT/INR measurements performed as inpatients or in the emergency room (ER) were excluded. If patients were instructed to not take their warfarin because of a procedure, INR values on those days were excluded from the study.
Data Collection
Data were collected by the primary investigator of this study after institutional review board approval to assess the impact of DMT. Demographic data, such as patient age and sex, and clinical information, such as diagnosis, warfarin dose, and medications, were collected to provide recommendations for patients based on their most current INR results. Retrospective chart reviews were performed to evaluate the efficacy of warfarin management through the use of a DMT by examining the TTR for the target and expanded therapeutic ranges (±0.2 and ±0.3 INR units), the average percentage of therapeutic INRs within the target and expanded therapeutic ranges, the total number of therapeutic INRs in both the target and expanded therapeutic ranges, and the total percentage of INR <1.5 and INR >4.5. An INR between 2 and 3 was considered the target range, representing adequate or therapeutic anticoagulation for all indications except aortic valve replacement (target INR range: 2.5–3.5).20 Since TTR is a measure of the quality of anticoagulation, TTR was calculated using the fraction of INRs method both pre- and post-DMT to assess the impact of this management process. TTR for the target range was calculated by dividing the number of INRs within the target range for all patients by the total number of INRs during the selected time interval.22 TTR for the expanded therapeutic ranges (±0.2 and ±0.3 INR units) was calculated by dividing the number of INRs within the corresponding expanded therapeutic target range for all patients by the total number of INRs during the selected time interval.3,12,22 These expanded therapeutic ranges have been shown to be more appropriate than the precise target range for assessing clinically significant INR control.3 The average percentage of therapeutic INRs in the target range was calculated by dividing the number of INRs within the target range by the total number of INRs during the selected time interval for each patient before and after implementation of the DMT. The average percentage of therapeutic INRs in the 2 expanded therapeutic ranges (±0.2 and ±0.3 INR units) was calculated in a similar manner, using the number of INRs within the corresponding expanded therapeutic range. Cutoff values for the expanded therapeutic range (±0.2 units) were 1.8–3.2 and 2.3–3.7 for the target INR ranges of 2–3 and 2.5–3.5, respectively. Similarly, for the expanded therapeutic range (±0.3 INR units), the cutoff values were 1.7–3.3 and 2.2–3.8 for these 2 target INR ranges, respectively.
Data Analysis
Data were processed using SPSS version 25 (SPSS INC., Chicago, IL) and Microsoft Excel version 2013 (Microsoft Corp., Redmond, WA). Paired-sample t-tests were used to compare the average percentage of therapeutic INRs in the target and expanded therapeutic ranges before and after DMT implementation. Similarly, paired-sample t tests were used to compare completion of quality performance measures related to warfarin management before and after implementation of the DMT. Finally, paired-sample t tests were used to compare the number of target and expanded therapeutic INR values before and after DMT implementation. The null hypotheses of this project are that the TTR for the target and expanded therapeutic ranges (±0.3 and ±0.2 INR units), the average percentage of target and expanded therapeutic INRs, the number of target and expanded therapeutic INR values, and the documentation performance measures (Table 1) will be similar before and after DMT implementation (H0 = 0). A P value <.05 was considered statistically significant.
The impact of DMT on warfarin management in terms of likelihood of bleeding and clotting were analyzed for those patients monitored before and after implementation of the DMT. It has been previously established that the likelihood of bleeding increases when INR is >4.5, and the likelihood of clotting increases when INR is <1.5.20
RESULTS
Patient Characteristics
A total of 50 patients were included in this study; 24 (48%) were male and 26 (52%) were female. Table 2 summarizes the age group, sex, INR goal, and warfarin indications for patients included in the study. A-fib was the most common indication for warfarin (48%), followed by DVT (28%), DVT/PE (6%), PE (8%), A-fib/DVT (2%), and aortic valve replacement (8%). The target range for 92% of the patients was 2–3. The other 8% of patients had a target range of 2.5–3.5 as seen in Table 2.
Patient characteristics
TTR
TTR was calculated as the fraction of INRs within the therapeutic range for both the target and expanded therapeutic ranges, as shown in Table 3. TTR for the target INR range was 52.19% before DMT implementation and 63.56% after DMT, representing an 11.37% point increase. TTR for the expanded therapeutic range (±0.2 INR units) was 63.90% before DMT and 77.33% after DMT, representing a 13.43% point increase. TTR for the expanded therapeutic INR (±0.3 INR units) was 68.78% before DMT and 81.38% after DMT, representing a 12.6% point increase.
Time in therapeutic range calculated using the fraction of INRs method
Average Percentage of Therapeutic INR Values
Table 4 shows the average percentage of therapeutic INRs in the target and expanded therapeutic ranges both before and after implementation of the DMT. The average percentage of INRs in the precise target therapeutic range was 61.99% before DMT implementation and 73.58% after DMT, which was a statistically significant increase (paired-sample t test, P = .030). The average percentage of therapeutic INRs in the expanded therapeutic range (±0.2 INR units) was 72.07% before DMT and 83.78% after DMT, which was also a statistically significant increase (P = .009). The average percentage of therapeutic INRs in the expanded therapeutic range (±0.3 INR units) was 75.55% before DMT and 86.2% after DMT; this was also a statistically significant increase (P = .009).
Average percentage of therapeutic INRs before and after diagnostic management implementation
Number of therapeutic INRs in the target and expanded therapeutic ranges
Percentage of INR Outside of Therapeutic Range
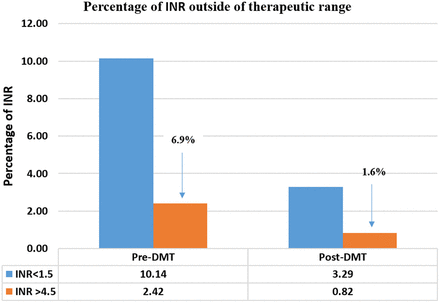
Figure 6 shows percentage of time within different INR ranges results (fraction of INRs): INR <1.5 and INR >4.5. After implementation of the DMT, the percentage for INR <1.5 was decreased by 6.85% points and the percentage for INR >4.5 was reduced by 1.6% points.
Percentage of INR outside of therapeutic range as determined by the fraction of INRs method (calculated by dividing the number of INRs within the range for all patients by the total number of INRs during the selected time interval).22 Percentage calculations for INR <1.5 and INR >4.5 are based on the total number of INRs before and after DMT implementation. DMT, diagnostic management team; INR, international normalized ratio.
Number of Therapeutic INR Values
The numbers of therapeutic INR values in the target and expanded therapeutic ranges before and after DMT implementation are shown in Table 3-5. The number of INR values in the precise target range was 107 before DMT implementation and 157 after DMT, which was a statistically significant increase (paired-sample t test, P < .001). The number of INRs in the expanded therapeutic range (±0.2 INR units) was 131 before DMT and 191 after DMT, which was also a significant increase (P < .001). Likewise, the number of INRs in the expanded therapeutic range (±0.3 INR units) was 141 before DMT and 201 after DMT, which was a significant increase (P = .001).
DISCUSSION
The results of this study demonstrated that implementation of a DMT directed by a DCLS-qualified professional at a rural hospital led to positive outcomes for patients and the institution. There were significant increases in TTRs for the target range, as well as the expanded therapeutic ranges (±0.2 and ±0.3 INR units), after DMT implementation. There were also significant increases in the percentage and number of therapeutic INRs for the target and expanded therapeutic ranges (±0.2 and ±0.3 INR units) from pre- to post-DMT.
TTR <60% is considered a marker of poor INR control and is indicative of patients with an increased risk of thrombotic and hemorrhagic events.23 Similarly, based on previously published studies, one can infer that by achieving a target TTR >70%, maximum benefits of warfarin may be expected, including fewer major bleeding events, thromboembolic complications, ER visits, and hospitalizations related to warfarin.11,24 In the current study, TTR values calculated using the fraction of INRs method for pre-DMT and post-DMT were 52.19% and 63.56% for the precise target range, respectively. Since the pre-DMT target TTR was <60%, this suggests that warfarin control was poor before DMT implementation, but it improved after DMT implementation. When considering TTR values for the expanded therapeutic ranges (±0.2 and ±0.3 INR units), the post-DMT TTR was >70% for both ranges, indicating that participants spent more time in the therapeutic range and were thereby potentially able to obtain the maximum benefits of warfarin therapy.
In a Canadian study, researchers conducted a randomized controlled trial in which patients were monitored by either by a pharmacist or family physician.25 TTR for the expanded therapeutic range (±0.3 INR units) was 82% for patients managed by a pharmacist, in contrast to 76% for those managed by a family physician.25 TTR after DMT implementation obtained in the current study for the expanded therapeutic range (±0.3 INR units) was 81.38%, which is consistent with the results of this previous study. Similarly, in a randomized control trial conducted in Hong Kong, patients receiving warfarin were randomized to either a pharmacist-managed service (n = 68) or a physician-managed service (n = 69).25,26 TTR for the precise target therapeutic range was 64% for the pharmacist-managed group and 59% physician-managed group, which were similar to the results obtained in our current study. A retrospective study conducted in an 8-county healthcare system in central New York State compared TTR for an expanded therapeutic range (±0.2 INR units) for 3 different models of anticoagulation management: usual care (physician-led), nurse-managed, or pharmacist-managed model.27 TTR was 49.4% with the usual care model, 67.3% with the nurse-managed model, and 74.9% with the pharmacist-managed model. In the present study, post-DMT TTR for the expanded therapeutic range TTR (±0.2 INR units) was 77.33%, which was comparable to the value for the pharmacist-managed model.
INR values outside the therapeutic range can lead to an increased risk of adverse patient outcomes. INRs <1.5 increase thrombotic risk in patients receiving warfarin, whereas INRs >4.5 increase the risk of hemorrhage (23). The percentage of time with an INR <1.5 (thrombotic risk) was lower post-DMT than pre-DMT. The percentage of time with an INR >4.5 (hemorrhagic risk) was also lower after DMT than before DMT. Both of these results suggest that implementation of DMT can lower the risk of both thrombotic and hemorrhagic events.
CONCLUSION
This study demonstrated that warfarin managed by a DMT collaborating with nurses and physician may be superior to other anticoagulation management service models. Using a standardized approach to warfarin management through a DMT significantly improved warfarin management and increased TTRs for both target and expanded therapeutic ranges. Rural healthcare systems with limited resources can utilize the DMT model with their current clinical staff to effectively manage patients receiving warfarin therapy.
Recommendations for Future Research
Our study has shown that a team-based approach improves therapeutic INR control in patients receiving warfarin. However, future studies that include a larger sample size are needed to address these issues more comprehensively and to evaluate the long-term effects of DMT on patient’s knowledge of warfarin, management, therapeutic outcomes, and complications. Future research may also examine the rates of anticoagulation-related ER visits and hospitalizations and the cost-effectiveness of DMTs for managing patients receiving warfarin therapy.
- Received January 25, 2024.
- Accepted March 10, 2024.
American Society for Clinical Laboratory Science