This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- East Central and Southern Africa Health Community
- East Central and Southern Africa Health Community
- Botswana-Harvard AIDS Institute Partnership
- Address for Correspondence: Talkmore Maruta
, East Central and Southern Africa Health Community, talkmoremaruta{at}gmail.com
ABSTRACT
The laboratory plays a critical role in the diagnosis and management of diseases. To ensure reliability, laboratories implement Laboratory Quality Management Systems (LQMS) and accreditation. Performance of 11 laboratories from 4 countries was monitored over 3 years using the World Health Organization/Regional Office for Africa Strengthening Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) process and a survey on perceptions of staff to identify barriers to implementation of LQMS. The mean SLIPTA score increased from 178 to 233 between 2017 and 2019, respectively. The 67 surveyed staff rated staff commitment (88%), staff knowledge of LQMS (78%), and management (84%) as adequate and staffing (60%) and financial resources (55%) as inadequate. Factors associated with achieving at least a 20% change in percentage score were: staff knowledge of LQMS [odds ratio (OR) = 0.06, 95% confidence interval (CI): 0.004–0.80; P = .034], management knowledge of LQMS (OR = 0.22, 95% CI: 0.06–0.80; P = .022), and workload (OR = 3.61, 95% CI: 1.41–9.22; P = .07). There was a significant association between achieving at least 20% change in scores and infrastructure (P = .05), knowledge of LQMS by laboratory staff (P = .01), and workload (P = .07). Knowledge of LQMS by laboratory staff and laboratory management, infrastructure, workload, and management support were potential barriers to the successful implementation of LQMS.
- AFRO - Regional Office for Africa
- CI - confidence interval
- ILAC - International Laboratory Accreditation Committee
- IQR - interquartile range
- ISO - International Organization for Standardization
- LQMS - Laboratory Quality Management Systems
- OR - odds ratio
- SATBHSS - Southern Africa TB and Health Systems Strengthening
- SD - standard deviation
- SLIPTA - Strengthening Laboratory Quality Improvement Process Towards Accreditation
- SLMTA - Strengthening Laboratory Management Towards Accreditation
- TB - tuberculosis
- WHO - World Health Organization
INTRODUCTION
The laboratory has increasingly played a key role in the diagnosis, management, and treatment of diseases within the health delivery system with 70% of clinical decisions depending upon or confirming medical laboratory test results.1 The increased investment in treatment and care through multilateral donor agencies and governments has resulted in expansion and strengthening of the public health system and saving of lives.2 Consequently, the demand for diagnostics has also increased to match the increased demand due to the expansion of the health system in general.
In order to meet the demand for testing as well as realize potential benefits of the public health investments like better patient outcomes, there has been an increased need to have reliable data from laboratories to inform decisions at diagnostic, monitoring, and policy level. Hence, quality laboratory testing has become critical in confirming the clinical diagnosis and provision of accurate disease surveillance data and directing public health care policy.3 To implement quality in a systematic way, laboratories use a quality management system approach in which policies, procedures, and processes are standardized and monitored for compliance to international standards like the International Organization for Standardization (ISO). One such standard specific for medical laboratories is the ISO 15189 standard for quality and competency.4
Compliance to international laboratory quality standards like ISO is a measure of the quality of data generated by the laboratory wherein quality is defined as the consistent generation of accurate, reliable, and timely results.
Accreditation is one means widely used by laboratories to confirm the implementation of quality management system consistently through an independent evaluation process offered by the International Laboratory Accreditation Committee (ILAC)-affiliated accreditation bodies.5
Through a voluntary process, the ILAC-affiliated accreditation bodies offer accreditation status as a means of confirming, through an independent evaluation process, compliance of a laboratory to international requirements for quality and competence like ISO. Although medical laboratories have long been involved in the implementation of a quality management system based upon the continuous improvement cycle, very few have attained international accreditation status.6 In April 2017, there were 485 laboratories with ISO accreditation in Africa, with 383 (79%) located in South Africa alone.7 The limited number of laboratories accredited and their concentrated location in South Africa are suggestive of systematic challenges in the implementation of quality management system in Africa.
The report on the implementation of the World Health Organization (WHO)/Regional Office for Africa (AFRO) Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) highlighted some notable challenges faced by countries implementing that program. These were related to technical areas including internal auditing and corrective action implementation processes.8 The authors recommended that further analysis was required on possible barriers to the implementation of quality management systems in Africa.8
As part of the regional efforts to strengthening health systems, the World Bank supported a regional project, Southern Africa TB and Health Systems Strengthening (SATBHSS), being implemented in 4 Southern African countries of Lesotho, Malawi, Mozambique, and Zambia. Among its objectives, the project supports strengthening laboratory systems through training and capacity building of staff, investments in laboratory infrastructure, strengthening sample transport networks, and implementation of Laboratory Quality Management Systems (LQMS) using Strengthening Laboratory Management Towards Accreditation (SLMTA) and WHO SLIPTA).9 Two of the laboratories enrolled in the project have received ISO accreditation status.
This paper describes the barriers to implementation of quality management systems toward ISO accreditation for the SATBHSS project country laboratories.
METHODOLOGY
Data were collected from the 13 laboratories on their performance using the WHO/AFRO SLIPTA checklist and the selected staff perceptions on how selected key aspects and requirements affected their implementation of LQMS using a survey questionnaire. The laboratories were from the countries of Lesotho, Malawi, Mozambique, and Zambia with a combination of national and district laboratories that were enrolled and supported through the SATBHSS project.
Measurement of Laboratory Performance
(a) Audit Process
A peer-to-peer regional audit process in which auditors conducted audits of laboratories in countries other than their own was used. For example, an audit team comprising auditors from Lesotho, Malawi, and Mozambique audited Zambia. This peer-to-peer approach was unique and ensured objectivity and transparency while encouraging peer learning and transfer of experiences across countries. The auditors were trained and certified as WHO/AFRO SLIPTA auditors by the African Society for Laboratory Medicine. Laboratories were audited yearly between 2017 and 2019.
(b) Audit Tool
Implementation of LQMS was measured using the scored WHO/AFRO SLIPTA checklist.10 This checklist allows for quantitative measurement of implementation of ISO 15189 standard for quality and competency and adherence to accreditation requirements. The scored checklist allowed for the laboratory performance to be rated using a 5-star scale of 0 to 5 stars. Each star level was associated with performance in terms of total scores in the assessment as follows: 0–150 points (<55%) = 0 stars, 151–177 points (55%–64%) = 1 star, 178–205 points (65%–74%) =
2 stars; 206–232 points (75%–84%) = 3 stars, 233–260 points (85%–94%) = 4 stars, and 261–275 (≥ 95%) points = 5 stars. Laboratories that attained and maintained ISO 15189 accreditation during the same period were then excluded from the yearly WHO/AFRO SLIPTA audits and considered as 5 stars equivalent in the final star rating analysis.
(c) Perception on Barriers to Implementation of LQMS
An online survey questionnaire developed by the authors was sent to 13 project laboratories to be completed by laboratory staff. The questionnaire was developed based on the findings from the implementation of the WHO/AFRO SLIPTA program and other studies that identified laboratory infrastructure, adequate and appropriately skilled human resources, and finances as some of the potential barriers to implementation of LQMS.10,11 The questionnaire submitted and returned via the online Survey Monkey collected information on laboratory staff opinion on how management support, staffing, infrastructure, financial resources, staff commitment, and workload affected the implementation of LQMS in their laboratories.
(d) Barriers to Implementation of LQMS
Descriptive statistics were used to analyze laboratory performance across the 12 Quality System Essentials using mean, standard deviation (SD), and interquartile ranges (IQRs) for absolute and percentages WHO/AFRO SLIPTA scores. Univariate logistic regression was used to assess for barriers to implementation of quality management systems by testing for predictors for successful performance in a WHO/AFRO SLIPTA audit using the variables from the survey questionnaire sent to laboratory staff. Successful performance was considered to be a change of at least 20% in the WHO/AFRO SLIPTA score in subsequent audits. The difference between each star level in the WHO/AFRO SLIPTA checklist (2015 version)10 is 10%, and an improvement by at least 2 WHO/AFRO SLIPTA star levels (20% change) was considered to be a substantial change in this analysis.
Sample Size Calculation
The sample size was calculated using Epi Info For Windows Version 7.2. Using laboratory information data collected during assessments on a number of staff in each laboratory, there were a total of 136 staff in the 11 laboratories. Using a 95% CI and 10% allowable error rate, the target sample size was 56.
RESULTS
(a) Participants and Laboratory Profiles
Of the 120 participants surveyed, 67 (56%) participants who responded to the survey were from Lesotho (16), Malawi (15), Mozambique (5), and Zambia (31). The participants were from 11 of the 13 (85%) SATBHSS project laboratories. Participants from 2 of the project laboratories were excluded because their laboratories did not have a complete set of 3 WHO/AFRO SLIPTA data points because of their unavailability for auditing at least once between 2017 and 2019. Participants included laboratory managers (19%), quality managers/officers (19%), and laboratory technicians (62%), respectively. The majority (87%) had been implementing LQMS for up to 3 years. Twenty-seven (40%) were working in a laboratory that became accredited between 2017 and 2019.
All the laboratories reported using SLMTA and on-site mentoring as tools for implementing LQMS. SLMTA is a competency-based training and mentoring program implemented across many laboratories in the Africa region that uses both workshop and work-based learning projects to effect immediate and measurable laboratory improvement.10 All laboratories received support to implement LQMS from a combination of government and implementing partners. The laboratory staff indicated workload was manageable (57%) and high (43%) during the period of implementation.
(b) Participants’ Rating of the Adequacy of Key Components of the Quality Management Systems
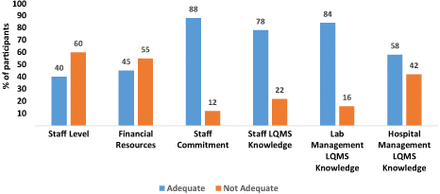
The 67 respondents rated the adequacy of the 6 variables of staffing level, availability of financial resources, commitment by laboratory staff, and laboratory and hospital management knowledge of LQMS during the implementation of LQMS. On a scale of 1–10, with 1 being not adequate and 10 adequate, the mean score rating was highest for laboratory management knowledge of LQMS (mean = 7.6, SD = 2.0) and lowest for availability of financial resources (mean = 5.4, SD = 2.7; Table 1).
Summary of ratings on adequacy of the six variables
The majority of participants rated staff commitment (88%), knowledge of LQMS by staff (78%), and laboratory management (84%) as adequate during the period of implementation of LQMS. However, 60% and 55% indicated staffing levels and availability of financial resources to support implementation of LQMS as not adequate (Figure 1).
Distribution of the participants rating of the key components of quality management in their laboratories.
(c) Laboratory Performance
The mean WHO/AFRO SLIPTA score increased from 178.2 in 2017 to 194.4 in 2018 and 233 in 2019, translating to an increase in WHO AFRO star levels from 2 (2017), 2 (2018), and 4 (2019) (Table 2). There was a mean change in scores of 21.9 (SD = 22.4, IQR = 7.5–41.5) between 2017 and 2018 and 58.3 (SD = 24.4, IQR = 60–69) between 2017 and 2019.
Performance of project laboratories in WHO/AFRO SLIPTA audits 2017–2019
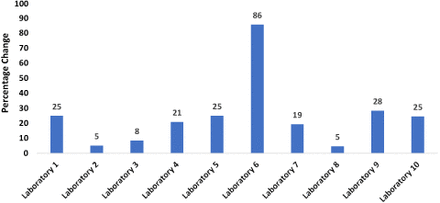
Over the project period 2017–2019, the maximum percentage change in WHO/AFRO SLIPTA score ranged from 5% to 86% (see Figure 2). Laboratories with very low percentage increase in scores had initial high baseline scores at the beginning of the project, and 2 of these were able to attain ISO accreditation status by 2019.
(d) Barriers to Implementation of LQMS
Each of the participants was requested to list up to 3 of what they perceived as the most important barriers to successful implementation of LQMS based on their experience in implementation of LQMS in their laboratory. Of the 195 barriers listed, management support was mentioned most (n = 32; 16%), followed by availability of reagents and supplies for testing (n = 29, 15%). Lack of training and knowledge of LQMS was the least mentioned (n = 14, 7%) as a barrier (Figure 3).
Distribution of the laboratories change of SLIPTA scores from 2017 to 2019.
Barriers to LQMS implementation as identified by participants.
(e) Predictors of Successful Implementation of LQMS
We sought to identify factors associated with significant improvement in LQMS implementation as measured by a change of at least 20% in SLIPTA score in subsequent audits. The difference between each star level in the WHO/AFRO SLIPTA checklist (2015 version) is 10%, and an improvement by at least 2 WHO/AFRO SLIPTA star levels (20% change) was considered as a significant change in this analysis. At univariate, factors that were associated with achieving at least a 20% change in percentage score were: knowledge of LQMS by laboratory staff (OR = 0.06, 95% CI: 0.004–0.80; P = .034), knowledge of LQMS by laboratory management (OR = 0.22, 95% CI: 0.06–0.80; P = .022), and workload (OR = 3.61, 95% CI: 1.41–9.22; P = .007).
Although not statistically significant, a laboratory with good infrastructure and adequate staffing were 5.4 and 1.7 times were more likely to achieve at least 20% change in WHO/AFRO SLIPTA score, respectively (Table 3).
Summary of predictors of successful implementation of LQMS
Using Chi-square analysis, there was a significant association between achieving at least a 20% change in scores and infrastructure (P = .05), knowledge of LQMS by laboratory staff (P = .01), and workload (P = .07). A trend moving toward significant association was shown with management support (P = .06) and knowledge of LQMS by laboratory management (P = .09). Based on the participant’s perception only, there was an association between workload and staffing levels (Chi-square = 8.61, P = .03).
DISCUSSION
Notably, all laboratories in the study used SLMTA and on-site mentorship as the tools for implementation of LQMS, which is consistent with the adoption of these tools by the 4 countries between 2010 and 2014.12 Over 197 laboratories were ISO accredited globally using the same tools.13 Under the SATBHSS project, the World Bank committed resources to support the 4 countries response to the tuberculosis (TB) pandemic including strengthening of TB diagnostics. It was therefore not surprising that most participants perceived laboratory support and staffing levels as adequate during the period of implementation of LQMS. Similarly, they perceived their knowledge of LQMS and that of laboratory management as adequate, which could be attributed to resources committed by the project to training and capacity building in LQMS training like understanding of ISO 15189, LQMS, mentorship, and quality improvement process training supported by the project.
Laboratory performance showed improvement from average 2-star rating in 2017 to 4 stars in 2019, with 3 of the laboratories achieving ISO 15189 accreditation by 2019. Given that selection of laboratories was based on the countries’ national strategic plan, prioritization of these laboratories for support and the guarantee of resources from the project could have contributed to the better performance.
Based on participant perception, management support, consistent availability of reagents, and supplies and laboratory infrastructure were among the barriers to the successful implementation of LQMS. Using a change in WHO/AFRO SLIPTA score of 20%, knowledge of LQMS by laboratory staff and laboratory management and workload predicted a change in WHO/AFRO SLIPTA score of at least 20%. Similarly, there was concordance with an analysis of the association between a change of at least 20% in WHO/SLIPTA score with knowledge of LQMS by laboratory staff (P = .01) and workload (P = .25, .13) and, in addition, infrastructure. Notably, although not statistically significant, there was a trend toward significant association with knowledge of LQMS by laboratory management (P = .06). These findings were consistent with the findings from the implementation of the WHO/AFRO SLIPTA program in 159 laboratories between 2013 and 2015, wherein country strategic planning, committed resources, infrastructure, human resources, and training in laboratory quality improvement were indicated as key factors to the attainment of at least 3 WHO/AFRO SLIPTA stars.8
Some of the prerequisites for enrolment into SLMTA include having a national laboratory policy, strategic plan, and commitment for financial and political support for the program.10 Given that all 11 laboratories were enrolled in SLMTA over the project period, factors that require commitment at the national and local level of laboratory management, training, and capacity building of laboratory personnel in LQMS and state of laboratory infrastructure were among the predictors of successfully implementing LQMS.
Since the laboratories in the study were project-targeted laboratories, their performance may not represent the other laboratories within the network that were not receiving special support and earmarked for accreditation by the national strategic plan. However, this study indicated that such high-level commitment and having a clear roadmap for accreditation is critical in the success of LQMS programs in the country.
Perception of participants was used as a basis for measurement, and that approach has been known to have limitations that include the reliability of the views that can be influenced by the desire by participants to bolster certain views.14 However, use of perception has also been shown to be able to measure intangible or difficult-to-measure issues like management support and staff motivation.14 In this study, the laboratory staff are the final recipients and implementers of the different interventions, and their perception on a number of key elements that determine success for implementation is valuable in providing insights on how programs like the implementation of LQMS can be optimized. To improve the quality of the findings, the perceptions by participants were cross-triangulated with quantifiable measures of laboratory performance using standardized tools like the WHO/AFRO SLIPTA checklist scores.
The period under consideration was 3 years, which could be considered as not long enough to observe the impact of the interventions by the project and correctly identify barriers. However, this review was also informed by the requirement of a midterm review by the project in order to inform areas for adjustment to maximize the investments being made. The concentrated efforts in these 11 laboratories also accelerate progress, as observed in the results, and in a way allow for rapid identification of barriers.
CONCLUSION
Knowledge of LQMS by laboratory staff who are implementing it and laboratory management supporting the implementation of the system, infrastructure, workload, and management support are potential barriers to the successful implementation of LQMS in public health laboratories.
- Received September 28, 2023.
- Accepted December 11, 2023.
American Society for Clinical Laboratory Science