This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Tim R. Randolph
, Saint Louis University, tim.randolph{at}health.slu.edu
ABSTRACT
Individuals with sickle cell trait (SCT) participate in competitive athletics and/or military training. Some have vigorously trained or competed without incident; whereas, others have encountered serious medical complications. Universal precautions are recommended to reduce the risk of exercise collapse associated with sickle cell trait (ECAST) and exercise-related death (ERD). However, there remain deaths in sport and military training attributed to SCT. ECAST and/or ERD are relatively unpredictable and are accompanied by rhabdomyolysis, thrombosis, hemolysis, renal dysfunction, and inflammation. Abnormal thrombotic markers have been reported in patients with SCT at rest. This pilot study measured total protein (TP; myolysis/hemolysis), creatine kinase (CK; myolysis), haptoglobin (hemolysis), dimerized plasmin fragment D (D-dimer), and fibrin monomers (thrombosis) at rest in 4 patients with SCT and 4 healthy patients at 4-time intervals at least 2 weeks apart. AS patients demonstrated lower haptoglobin, higher TP, and higher CK levels compared with AA patients, suggesting subclinical hemolysis and myocyte destruction at rest. Findings for the D-dimer and fibrin monomer tests were positive in 56.3% and 12.5%, respectively, of SCT patients and negative for all AA patients. The SCT patients demonstrated day-to-day variability of D-dimer and fibrin monomer levels with positive test results on some, but not all, of the 4 testing occasions. These data support previous findings that SCT patients exhibit thrombotic activity at rest and suggest the potential for hemolysis and myolysis. These abnormal biomarkers may prove useful to predict risk (baseline values), identify responders to exercise, or identify early-onset ECAST (during exercise) to reduce exertion-related adverse events.
- CK - creatine kinase
- CRP - C-reactive protein
- D-dimer - dimerized plasmin fragment D
- ECAST - exercise collapse associated with sickle cell trait
- ERD - exercise-related death
- NCAA - National Collegiate Athletic Association
- SCD - sickle cell disease
- SCT - sickle cell trait
- TP - total protein
INTRODUCTION
Sickle cell anemia was first reported in 1910 by James B. Herrick, a physician practicing in Chicago, who described a 20-year-old Black Caribbean dental student as having shortness of breath, palpitations, yellow eyes, and anemia.1 Once the disease was characterized as a single gene mutation, homozygotes were described as having sickle cell disease (SCD); whereas, heterozygotes were thought to be benign carriers and given the designation sickle cell trait (SCT). SCD is estimated to affect 100 000 US citizens: 1 in every 365 African American births and 1 in 16 300 Hispanic American births.2,3 The prevalence of SCT among African Americans in the United States is about 8% (1 in 13 African American births), representing 3 million affected Americans and 300 million worldwide.4⇓-6 Much research has been done on SCD, but in the 1970s and early 1980s, observations were published suggesting that individuals with SCT might be better classified as having a mild clinical condition.4,7⇓-9 After much debate, the conversation resurfaced 30 years later.5,6,10,11 The observations suggesting that SCT may be a clinical condition occurred within 2 primary groups, warfighters and athletes, and in 2 primary forms, exercise-related death (ERD) events and long-standing, subclinical health concerns.
The first report of ERD among US warfighters with SCT was in 1970 at Fort Bliss, Texas, when 4 Army recruits died during basic training. These 4 recruits were among the 2% of all recruits that year with SCT, accounting for 1 death for every 600 recruits with SCT. Among the 5 reported warfighters who died of exercise-related events from 1965–1981, 4 had SCT; whereas, the fifth was a compound heterozygote (sickle cell hemoglobin C).12,13 In 1987, Kark et al12 reported that 7.8% of military recruits had SCT and described the association between strenuous exercise and the risk of sudden death among soldiers with SCT. The risk of nontraumatic sudden death in Black recruits was 32-fold higher than non-Black recruits and 27.6-fold higher for Black recruits with SCT compared with Black recruits without SCT.12 Among recruits with SCT, the rate of ERD was 11–30/100 000 recruit training cycles compared with 1/100 000 recruit training cycles without SCT. This risk normalized after basic training for 3 primary reasons: (1) most new recruits enter basic training without previous physical conditioning putting them at risk; (2) physical demands lessen after basic training, reducing the risk; and (3) those who experience exercise-related events discontinue service selecting for those with lower risk. Physiologically, exercise-related events produce hypoxia and acidosis, which promotes sickling, leading to death primarily from rhabdomyolysis, heat stroke, and cardiac arrythmias.13
Sudden death associated with SCT was first reported on the athletic field in 1974, involving an American collegiate football player.13⇓-15 Although sudden death has been observed in a variety of sports, football has produced the most reports and garnered the most press. Similar to the military, the prevalence of professional football players with SCT was reported to be 6.7% in 1973, consistent with the general population.16 National Collegiate Athletic Association (NCAA) Division I football athletes with SCT are estimated at 3%–4%17 and have a relative risk of death that is 37 times greater than those without SCT; this risk is 15 times greater when considering all NCAA sports.18 It is estimated that approximately 5300 NCAA athletes across all sports have SCT.17 Between 2000 and 2010, 16 deaths occurred in NCAA Division I football, and all 16 were during conditioning drills; none were during games. Of these 16 deaths, 4 were classified as cardiac, 1 was asthma, 1 was exertional heat stroke, and 10 (63%) were associated with complications of exertional sickling.17
In 2012, O’Connor et al19 assembled military, athletic, and hematology experts, resulting in a publication describing guidelines to mitigate the risks of exercise collapse associated with SCT (ECAST) for warfighters and athletes. Also in 2012, the National Athletic Trainers Association published practice guidelines for the care of athletes with SCT that focused on universal precautions for all athletes along with specific guidelines for those with SCT to include an awareness of symptoms of rhabdomyolysis and sickling for the individual and all those present during conditioning sessions.20
In addition to ECAST, a wide variety of health concerns were also reported in individuals with SCT. Clinical complications with a “definite” connection to SCT have been described as renal medullary carcinoma, hematuria, renal papillary necrosis, hyposthenuria, and splenic infarction. Other clinical issues that have “probable” associations with SCT include complicated hyphema, venous thromboembolism, fetal loss/demise, and low birth weight. Clinical conditions with a “weak or possible” association with SCT are acute chest syndrome, asymptomatic bacteremia in pregnancy, and proliferative retinopathy.10
Hypercoagulable state is present in patients with SCT,21 and the level of activation increases with disease severity from AS to SC to SS.22 Thrombotic activation in individuals with SCT contributes to the risk of thromboembolism.23,24 Elevated dimerized plasmin fragment Ds (D-dimers), thrombin/antithrombin complexes, and fibrinogen fragment 1.2 have been reported in AS patients at rest, confirming baseline thrombotic activity and providing potential biomarkers to identify thrombotic activation.21,25
SCT is now recognized as a contributing factor for ECAST and ERD in warfighters and athletes and poses potential health risks for all who bear the mutated gene. Rhabdomyolysis is the primary pathophysiologic event present in ECAST and ERD,4⇓-6,9⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-20,26 but thrombosis,21⇓⇓⇓-25 sickling,12⇓⇓⇓⇓⇓⇓⇓-20 renal dysfunction,27⇓-29 inflammation,30⇓-32 and oxidative stress33⇓-35 have also been reported. However, ECAST and/or ERD are relatively unpredictable events. The concept of the perfect storm has been postulated retrospectively; whereas, activation of 1 or more of these pathophysiologic pathways in combination with dehydration, exercise intensity, diet, and a history of rhabdomyolysis contribute to ECAST, although the relationship of these factors is imperfect.36 It would be ideal if the risk of a perfect storm could be forecasted for an individual prior to participation.
Because thrombosis activation has been documented in SCT patients at rest, and it increases with disease severity, it is possible that subclinical activation might predict risk of ECAST. Similarly, if blood or urine biomarkers for myolysis, hemolysis from sickling, renal dysfunction, or inflammation could be identified that are also elevated at rest, a risk profile for ECAST may be possible. A mechanism to predict risk of ECAST can equip athletic trainers, military supervisors, and overseeing physicians with tools to reduce adverse outcomes and provide equal opportunity for individuals with SCT to serve in the military and compete in athletics.
The purposes of this pilot study were to corroborate finding of activated coagulation at rest in SCT patients, extend findings to biomarkers that assess pathophysiologic pathways other than thrombosis, and evaluate variability of abnormal baseline biomarkers over time.
MATERIALS AND METHODS
Research Participants
There were 4 patients with SCT and 4 healthy patients without SCT who consented according to the institutional review board and enrolled in the study. All patients were between the ages of 18 and 60 years, and no ongoing health issues were self-reported.
Sample Collection
Blood and urine samples were collected from each patient on 4 different occasions at least 2 weeks apart. At the first collection session, 3 tubes of blood were collected (EDTA, red top tube, sodium citrate) using standard venipuncture techniques along with 1 random urine sample. For collection sessions #2, #3, and #4, 2 blood tubes were collected (red top tubes and sodium citrate) with a random urine sample. Urine testing was performed within 2 hours of collection, blood samples were processed, and serum/plasma samples were stored at −18 °C for future batch testing.
Sample Testing
The EDTA blood samples from the first collection were used to verify the sickle cell hemoglobin genotype (AA or AS) using a hemoglobin solubility screen (Sickle Confirm [in-house method]) and hemoglobin electrophoresis confirmation (QuickGel [Helena Laboratories, Beaumont, TX]).
Plasma from the sodium citrate tube was used to test for D-dimer (latex agglutination [Remel, Thermo Fisher Scientific, Waltham, MA]) and fibrin monomer (hemagglutination [Diagnostica Stago Inc, Parsippany, NJ]).
Serum samples were tested for total protein (TP; dye binding) and creatine kinase (CK; enzymatic method) using the ACE Alera clinical chemistry analyzer (Alfa Wassermann, West Caldwell, NJ). Haptoglobin was measured by radial immunodiffusion (Kent Laboratories, Bellingham, WA).
A chemical urinalysis (dipstick) and a microalbumin (dipstick) were performed on each sample using the CLIAWaived Inc Auto Urinalysis Reagent test strips and the CLIAWaived Inc microalbumin 2–1 combo strips, respectively.
RESULTS
Mean values for the serum analytes—CK, TP, and haptoglobin—were compared between the HbAS (SCT) group and HbAA (normal control) group. Each collection time point was managed independently, creating 16 data points for each group. Mean values were plotted with 95% CIs and statistically analyzed using an independent T-test with the alpha set at 0.05.
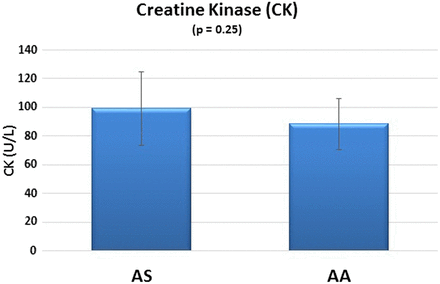
The AS group produced higher serum CK levels compared with the control group, but the difference was not statistically significant (P = 0.25). The 95% CIs suggest a greater variability in CK levels in the AS group compared with the AA group (Figure 1).
CK biomarkers in SCT.
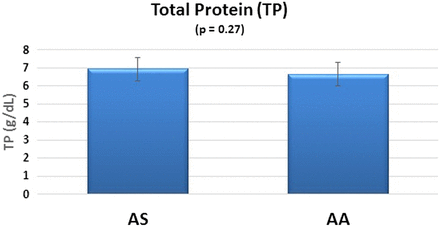
The AS group produced higher serum TP levels compared with the control group, but the difference was not statistically significant (P = 0.27). The 95% CIs show similar variability between the AS and AA groups (Figure 2).
TP biomarkers in SCT.
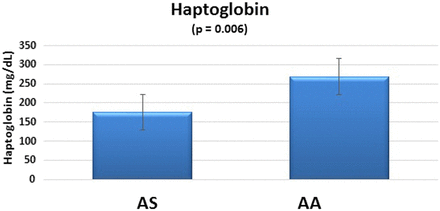
As can be seen in Figure 3, the AS group produced lower serum haptoglobin levels compared with the control group, and the difference was statistically significant (P = 0.006). The 95% CIs show similar variability in the AS and AA groups.
Haptoglobin biomarkers in SCT.
D-dimer and fibrin monomer were performed on citrated plasma from the 16 samples in each group. Results were recorded as positive or negative, and samples positive for D-dimer were diluted and retested to estimate concentration.
At least 1 positive D-dimer result was found in 3 of 4 AS patients with 1 AS patient showing elevated D-dimers at all 4 time points (Table 1). Overall, 9 of 16 (56.3%) AS blood samples produced a positive D-dimer result. A positive fibrin monomer result was produced in 2 of 4 AS patients, 1 positive result each among the 4 time points, totaling 2 of 16 positive result samples (12.5%). Interestingly, the AS patient with normal D-dimer levels at all 4 time points produced a positive fibrin monomer result. The other AS patient with a positive fibrin monomer result had a positive D-dimer result in all 4 samples collected. All AA patients in the control group had negative (normal) D-dimer and fibrin monomer results at all 4 time points.
Positive D-dimer and fibrin monomer results
Samples with positive D-dimer results were diluted to produce a semiquantitative concentration for each analyte. Table 2 shows the estimated levels of D-dimer for each positive result. D-dimer levels in positive-result AS samples ranged from 1–2 μg/mL to 4–8 μg/mL.
Estimated concentration of D-dimer in positive-result AS samples
Between-sample variability was observed for both the D-dimer and fibrin monomer levels because some AS patients had positive results and some had negative results. Likewise, within-sample variability was also observed because some time points produced a positive result for the D-dimer or fibrin monomer test and other time points produced a negative result.
A urine sample was collected on each patient in both groups at all 4 time points, and a chemical urinalysis (dipstick) was performed along with a microalbumin analysis. No abnormal results were obtained for any patient, at any time interval, in either group with 1 exception. An AS patient showed a positive urine leukocyte result from a slightly cloudy urine sample and self-reported symptoms of a mild urinary tract infection.
DISCUSSION
The primary aims of this pilot study were to confirm that some patients with SCT will show evidence of subclinical thrombotic activity by virtue of elevated D-dimer and/or fibrin monomer levels at rest and to test other blood and urine biomarkers for similar baseline abnormalities. In addition to testing for subclinical thrombotic activity, we were also interested in the possibility of subclinical myolysis, hemolysis, and renal dysfunction. We sought to screen for subclinical myolysis activity by detecting elevated CK in the blood and elevated blood and/or protein (both caused by myoglobin) in the urine. Subclinical hemolysis was evaluated in blood samples by measuring a decrease in haptoglobin and in the urine by detecting elevated blood and/or protein (both caused by hemoglobin). Renal dysfunction was evaluated by detecting any type of abnormality in the chemical urinalysis with an emphasis in blood, protein, and specific gravity on the dipstick.
Although not statistically significant, CK and TP levels were higher in the AS population compared with the AA control group. CK is an enzyme found in various tissues but is highest in muscle and brain. One specific isotype, CK-2 (creatine kinase MB), is highest in skeletal muscle tissue and is released upon skeletal muscle injury. An elevation of CK in the AS group is consistent with subclinical myolysis. Similarly, during bouts of intravascular hemolysis, hemoglobin is released from erythrocytes into the blood. Both CK and hemoglobin, if released in sufficient amounts from myocytes and erythrocytes, respectively, could produce an elevated TP as seen in the AS group. It is unclear if the statistically insignificant difference between groups is clinically insignificant and explained by random variability or if a larger sample size might produce statistically significant differences.
A statistically significant difference in blood haptoglobin levels between groups was observed. During episodes of intravascular hemolysis, the hemoglobin released from erythrocytes will bind to haptoglobin to be transported to the liver for detoxification and excretion. Once at the liver, haptoglobin releases the hemoglobin, which is converted to bilirubin, delivered to the gall bladder as a component of bile, and eventually excreted in the feces as urobilinogen. Lower blood haptoglobin levels in the AS group suggest subclinical intravascular hemolysis.
Elevated D-dimers and fibrin monomers corroborate previously reported thrombotic activity at rest in patients with SCT25 and showed 2 important findings related to subclinical thrombotic activation. First, the frequency of D-dimer (56.3%) and fibrin monomer (12.5%) elevations suggest that thrombotic activation is a viable marker to study further with potential to predict or identify ECAST. Second, the within-sample variability of positive results suggests that risk of ECAST may change day-to-day in AS patients, presumably in response to external factors that influence thrombotic activation. This variability may require ongoing screening prior to each exercise event by those caring for athletes and warfighters prone to ECAST. AS patient #4, a Black female in her 50s, had elevated D-dimer levels at all 4 blood samplings. Interestingly, AS patient #1, also a Black female in her 50s, demonstrated positive D-dimer results at 3 of 4 blood samplings. In contrast, AS patient #3, a Black female in her 30s, showed 2 positive D-dimer results, whereas AS patient #2, a Black female in her 20s, had no elevated D-dimer levels at any of the 4 samplings.
Funding and patient recruitment limitations prohibited assessment of a full range of potential biomarkers. Several biomarkers, other than those chosen in this study, are available to measure myolysis (myoglobin), thrombotic activation (thrombin/antithrombin complexes, fibrinogen fragment 1.2, protein C, and protein S), hemolysis (plasma hemoglobin and reticulocytes), renal dysfunction (serum urea nitrogen, creatinine, and creatinine clearance), inflammation (C-reactive protein [CRP], highly sensitive CRP, and ferritin), and oxidative stress (advanced oxidation protein products, malondialdehyde, nitrotyrosine, ferric-reducing antioxidant power, nitrite, and nitrate).
Future studies will include assessing (1) a wider selection of biomarkers on patients with SCT at rest to determine those most sensitive to subclinical pathophysiologic activations, (2) biomarker levels after moderate exercise to identify responders who show greater postexercise elevations compared with baseline, and (3) biomarker levels over time to verify within- and between-sample variability. Assessing abnormal biomarkers at rest will further inform subclinical pathophysiology and expand biomarkers that may be useful in clinical practice. Biomarkers that elevate following exercise presume a greater risk for ECAST and identify biomarkers to potentially predict risk and identify responders to exercise. If exercise responders also show baseline biomarker elevations, resting measurements of these biomarkers may be useful to predict responders. An ability to predict responders will equip health care providers with tools to counsel individuals about risk, customize workout routines to minimize risk, and identify early stages of ECAST, which together may reduce ECAST and ERD.
- Received March 15, 2019.
- Accepted June 3, 2019.
American Society for Clinical Laboratory Science