This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Chesley Kemp
, Augusta University, ches2496{at}gmail.com
ABSTRACT
The CellaVision DM9600 (CV) is an automated digital microscopy system that performs peripheral blood cell differentials. Manual differentials are prone to variable results between laboratory scientists (LSs). The CV may reduce this subjectivity, especially in the classification of reactive lymphocytes (RLs). The first aim was to investigate the correlation between CV and LS classification of RLs. The second aim was to determine whether the LS performing the reclassification affects the difference in the number of RLs classified between the CV and LS. The sample identified 3925 CV differentials completed between January 2018 and July 2019 with RLs identified by the CV, LS, or both. A related-samples Wilcoxon signed-rank test showed a statistically significant difference (P < .001) between the median number of RLs classified by CV and LSs. Spearman’s Rho showed no statistically significant correlation (P = .455). An independent-samples Kruskal-Wallis test showed a significant difference between LSs (P < .001). Dunn analysis further described the variability between LSs. Although there is no real correlation between CV and LSs’ classification of RLs, the difference between CV and LS is influenced by which LS is performing the analysis. Future studies may investigate factors influencing RL classification among LSs.
- CellaVision DM9600
- hematology
- lymphocyte subsets
- clinical laboratory techniques
- differential leukocyte count
INTRODUCTION
Review of peripheral blood smears is an invaluable part of patient diagnosis. In clinical laboratories, hematology analyzers perform automated cell counts and white blood cell differentials on patient samples. If the analyzer detects abnormal cells, it flags the differential and reflexes to manual peripheral blood smear analysis by a laboratory scientist (LS) to confirm the results.1 Estimates for the percent of samples flagged for manual review vary based on the study and the patient population, but as many as a quarter of peripheral blood smears may be flagged for manual review.2,3
Manual review of peripheral blood smears is a time-consuming process, especially for novice LSs.4 Besides the amount of time required for manual review, classification of certain cell types, such as reactive lymphocytes (RLs), may be highly subjective.5 As the population of the United States ages, the number of abnormal blood samples will only increase, and the necessity for manual peripheral blood review will increase with it.2
The introduction of the CellaVision DM9600 (CV; CellaVision AB, Lund, Sweden) has expedited the performance of white blood cell differentials in many clinical laboratories. The CV is a digital microscope with an automated pattern recognition system.1 During its operation, the CV performs white blood cell differentials by taking pictures of a predetermined number of cells at high magnification. The CV utilizes an artificial neural network to preclassify these cells into 17 cell types. An LS will then review the CV preclassifications for accuracy and reclassify cells as needed. After reclassification, the LS releases the results.6
Before reclassification, the CV preclassification of cells such as neutrophils and lymphocytes generally has a high correlation with manual review.2,4 For cells present in small numbers, such as eosinophils, monocytes, and basophils, correlation with manual review may decrease considerably.4,7 Basophils may be misclassified as much as 90% of the time.7 Immature granulocytes and blasts also frequently require reclassification.2,8 Reclassification produces results comparable with manual review.4,7
Despite the need for frequent reclassification of certain cell types, the time required for peripheral blood analysis by the CV is similar to or shorter than the time required for manual microscopic review. This includes the time required to review and reclassify results.4 The differences between the CV and manual review times are even more dramatic with novice LSs. Briggs et al4 observed a 75% reduction in time to review when using the CV as compared to manual review for 1 novice LS. The potential of the CV is an efficient and accurate automated method of microscopic blood review that will alleviate some workload for LSs.
One area in which the CV may be valuable is in the classification of RLs. Currently, limited research is available on CV classification of RLs. With manual white blood cell differentials, these cells are prone to inconsistent classification between LSs.5 Although reactive lymphocytosis is a nonspecific indicator of infection, RLs may be difficult to differentiate from other cells types, such as lymphoma cells. As a result, it is important to accurately identify RLs to distinguish benign proliferation from a malignant condition.9 Despite the importance of distinguishing RLs from other lymphocyte subtypes, differentiation between these subtypes is highly subjective.
A study by van der Meer et al5 found that in a white blood cell differential including 56 lymphocytes, only 7 lymphocytes were consistently classified by participants. The other 49 lymphocytes received a variety of classifications, from normal lymphocyte to atypical lymphocyte to plasma cell. In the case of 1 lymphocyte that was contained twice in the survey, 31% of LSs failed to classify the cell the same way both times. Other publications support the presence of interobserver discordance in classifying RLs.10⇓-12 If the CV can accurately classify RLs, it could provide a more objective and consistent evaluation of these cells’ presence in a peripheral blood smear.
The first aim of this study was to determine if there was a correlation in the number of RLs classified between CV and LS. The second aim was to determine whether the LS performing the reclassification affects the difference in the number of RLs classified between the CV and LS.
MATERIALS AND METHODS
For clarity, “preclassified” will be used interchangeably with “CV classification,” and “reclassified” will be used interchangeably with “LS classification.”
The study was designed as a retrospective analysis of peripheral blood smears analyzed by the CV at the Augusta University Medical Center Core Laboratory during the course of normal laboratory operations. The dataset was composed of all the white blood cell differentials analyzed by the CV between the months of January 2018 and July 2019—a total of 19 months of differentials.
Only differentials in which RLs were identified by the CV, LS, or both were included for analysis. CV identification of RLs was determined by an artificial neural network.6 LS identification of RLs was determined following laboratory procedure. Differentials with no RLs identified by the CV or the LS were excluded from analysis. Data collected from each differential consisted of the performing LS, CV’s preclassified differential results, and LS’s reclassified differential results. The performing LS was identified based on the instrument log-on credentials. Initially, 26 unique LSs were identified based on the log-on credentials and deidentified by assigning an alphabet letter “A” through “Z.” One LS had 2 log-on credentials representing “L” and “Y”; these 2 were combined into “L” for analysis. LS “H” was the log-on used on the main computer connected to the CV to which all LSs had access; it was excluded from analysis. The final number of LSs for data analysis was 24. Each CV differential was associated with a single LS. In addition, each differential was deidentified of all patient information and assigned a number.
Shapiro-Wilk analysis was used to determine a nonnormal data distribution and to select the appropriate statistical analyses. A related-samples Wilcoxon signed-rank test was performed to determine whether the difference in the number of preclassified and reclassified RLs was significant. Spearman’s Rho analysis was performed to determine the correlation between the preclassified and reclassified results. An independent-samples Kruskal-Wallis test was used to determine whether the reclassifying LS affected the median difference between the preclassified and reclassified results. For the Kruskal-Wallis test, LS was the independent variable, and the difference between preclassified and reclassified results for each differential was the dependent variable. Dunn’s 1964 procedure with Bonferroni correction for multiple comparisons was performed to compare the median difference of CV and LS between LSs. IBM SPSS (version 27) was used for all analyses. R (version 4.2.1) was used to generate multiple figures.
RESULTS
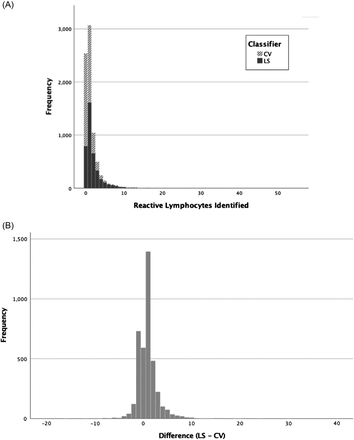
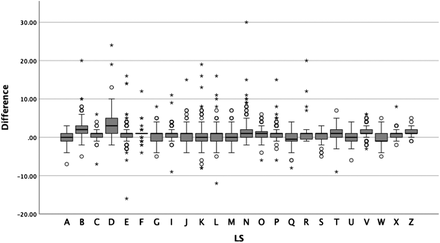
The sample consisted of 3925 unique white blood cell differentials containing RLs (Figure 1A). The CV preclassified a median of 1 (interquartile range [IQR], 1) RL; the range was 0–31. LSs reclassified a median of 1 (IQR, 1) RL; the range was 0–51. The distribution of number of RLs classified was shown to have a nonnormal distribution by Shapiro-Wilk analysis (P < .001). For 61% of differentials, LSs identified more RLs than the CV. LSs identified fewer in 24% of differentials. LSs and CV identified the same number of RLs in 15% of differentials. The median difference between LS and CV across all LSs was 1 (IQR, 2); the range was −16 to 30. (Figure 1B). The difference between the CV and LS could fluctuate considerably for a single LS, as indicated by the interquartile ranges (Figure 2). Spearman’s Rho showed no statistically significant correlation between the CV and LS (rs = 0.012, P = .455). A Wilcoxon signed-rank test showed a statistically significant median increase in the number of RLs identified by LSs when compared to the CV (z = 27.04, P < .001).
Histograms for RLs identified by CV, LS, and differences. (A) Number of RLs in a smear identified by CV and LS. (B) Difference in RLs identified by CV and LS.
Box plot of differences in RLs between CellaVision DM9600 and laboratory scientist. Points indicated by asterisk (*) are extreme outliers (3 × IQR). Points indicated by degree (°) are mild outliers (1.5 × IQR).
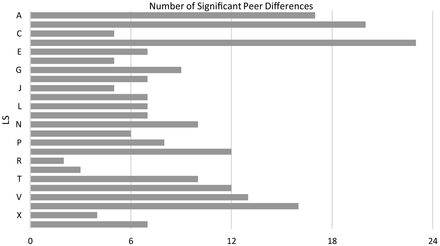
The independent-samples Kruskal-Wallis Test showed that the median difference between CLS and CV was significantly different between LSs (C2(23) = 592.5, P < .001). Dunn’s procedure compared the median difference for each individual LS to all of the other LSs and showed which LSs were significantly different from each other (Figure 3). Instances in which the median difference of 1 LS was significantly different from another LS were designated “significant peer differences.”
Peer differences. Number of statistically significant peer differences (P < .05) in median difference between CV and LS for each LS, as determined by Dunn’s procedure.
DISCUSSION
The lack of a correlation between CV and LS classification of RLs suggests the CV alone, without LS reclassification, is not a reliable method of classifying RLs. Generally, LSs identified more RLs than the CV (Figure 1B). As a result, it is possible that the CV’s artificial neural network is too conservative when identifying RLs. Similar underestimations of cell concentrations were previously an issue in blast classification. However, updates to the software increased CV recognition of blasts.8 Similar updates may be advisable for RL classification.
Although the CV may underestimate the number of RLs, the Kruskal-Wallis analysis indicates that the difference between LS and CV is dependent on which LS is performing the reclassification. Visually, this can be observed in Figure 3, which was generated from the results of the Dunn analysis. Some LSs, namely A, B, D, and W, had many significant (P < .05) peer differences, whereas others, like R and S, had only a few.
The reasons for these differences between LSs are unclear. A wide variety of factors could be at work, such as LS educational background and experience. The variable interquartile ranges for each LS and the number of outliers (Figure 2) suggest that the smears themselves and the inherent nature of RL variation in morphology between patients and clinical conditions may be factors influencing the difference between LSs. Some LSs may have received peripheral blood smears with more abnormal white blood cells, such as differentials from cancer patients undergoing treatment. Certain work shifts may encounter abnormal peripheral blood smears more frequently or involve different amounts of time to devote to reclassification. In future studies, LSs should evaluate the same smears to avoid this issue. Finally, some patients may be represented multiple times in the dataset. However, because the differentials were deidentified from patient information, the frequency of this occurrence cannot be determined.
With updates to the technology, as in Eilertsen et al8 with blast identification, the CV may eventually classify RLs more objectively and accurately than LSs. Multiple studies have indicated that there is little agreement between LSs when classifying lymphocytes into subclasses.5,10⇓-12 The study by Briggs et al4 also suggested that for certain cell types, preclassification may be more accurate than reclassification. In that study, LS reclassification generally had equal or greater similarity to the reference method when compared to preclassification. However, for certain cell types—mainly classes of immature neutrophils—and for specific LSs, the preclassified results had a stronger correlation with the reference method than reclassified results. This suggests that LS experience and cell type may influence the degree of correlation with the reference method for reclassified and preclassified results, and in some cases the CV alone may be more reliable than the LS. RLs could be 1 such case, given the preexisting degree of variability in their classification.5
The retrospective nature of this study was beneficial in that it allowed analysis of a large sample of white blood cell differentials with many reclassifying LSs. However, it precluded the use of a reference method to compare the accuracy of preclassification and reclassification. Any future studies would ideally compare preclassified and reclassified results individually to reference differentials performed by experienced LSs, similar to the method seen in Briggs et al.4 Data could also be collected regarding LS experience, such as educational background, number of years worked in the field, and level of certification, to determine how these factors influence RL classification.
Although RLs are important primarily to rule out other diagnoses, understanding how the CV and LS analyze these cells may provide insight into the classification of other white blood cells and lymphocyte subclasses. If there was a strong correlation between the CV and LS, reclassification might be unnecessary for RLs. It would also suggest that the CV may be reliable in identifying other lymphocyte subtypes. Elimination of reclassification would further streamline the process of peripheral blood review in the clinical laboratory. It would increase the objectivity when classifying cells prone to variable identification because it avoids factors influencing LS decisions, such as work experience. By reducing the time required for peripheral blood review and increasing the consistency of results, the work burden on LSs may be decreased, and better patient outcomes may be obtained.
- Received June 22, 2024.
- Accepted September 10, 2024.
American Society for Clinical Laboratory Science