This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Stephanie L Mitchell
, University of Pittsburg School of Medicine, mitchellsl5{at}upmc.edu
LEARNING OBJECTIVES
1. Define metagenomics and possible applications in the clinical microbiology laboratory.
2. Discuss the advantages and limitations of metagenomic next-generation sequencing (mNGS) diagnostics.
3. List the skillsets needed for wet- and dry-bench laboratory personnel who perform mNGS assays.
ABSTRACT
Next-generation sequencing (NGS)-based assays have recently entered the realm of the clinical microbiology laboratory’s capacity, providing exciting potential for improvement in infectious disease detection and identification. There are many diagnostic applications of NGS, such as targeted or amplicon NGS and metagenomic NGS (mNGS). mNGS has received the most attention for diagnostics because of its unbiased nature and “hypothesis-free” testing approach. Although mNGS may have improved pathogen detection compared with conventional culture-based testing and has shown clinical utility in some specific cases, the application of this technology is still investigational, and many barriers and limitations remain to be overcome. This review will cover both the advantages and limitations of mNGS and address the need for and incorporation of new technologist skillsets in the clinical microbiology laboratory to successfully implement mNGS diagnostics.
- BAL - bronchoalveolar lavage
- cfDNA - cell-free DNA
- CMV - cytomegalovirus
- CSF - cerebral spinal fluid
- GI - gastrointestinal
- HSV - herpes simplex virus
- IFI - invasive fungal infection
- mNGS - metagenomic next-generation sequencing
- NGS - next generation sequencing
- OAI - osteoarticular infection
- PCR - polymerase chain reaction
INTRODUCTION
Next-generation sequencing (NGS) is a technology that allows simultaneous, massively parallel sequencing of millions to billions of nucleic acid fragments.1 Although there are many clinical and research uses of NGS, metagenomic analysis of NGS data (also known as shotgun NGS and colloquially referred to as “mNGS”) is a highly sought-after application because of the ability to unbiasedly interrogate a sample for all groups of pathogens. This so-called “hypothesis-free” diagnostic approach can, in theory, detect any bacteria, virus, fungi, and/or parasite directly from patient samples.1 Although traditional culture remains the gold standard, mNGS has been shown to provide improved pathogen detection compared with culture, especially for difficult-to-culture and unexpected pathogens.2 Numerous case reports that highlight the advantage of mNGS for clinical diagnosis have been reviewed elsewhere.3 However, there are significant limitations and hurdles when applying mNGS to clinical testing that should be understood to grasp the full potential and utility of this new approach. This review aims to highlight the advantages and disadvantages of mNGS and considerations for implementing this method in infectious disease clinical diagnostics with specific focus on associated workforce needs.
THE CLINICAL LABORATORY APPROACH TO mNGS IMPLEMENTATION
Prior to adopting mNGS in the clinical laboratory, one must first determine if the laboratory is able to support the technology. Wet-bench technologists should have expertise in molecular techniques and be accustomed and adhere to proper molecular practices. Proper personal protective equipment, sample handling, and a unidirectional laboratory workflow should be familiar to those engaged in sample extraction, library preparation, loading, and running NGS platforms. Preparation of the sample and the NGS library are currently labor-intensive and require multiple high-complexity steps.4 Depending on available staff and laboratory workflow, wet-bench processes can take up to 2–3 days. DNA library preparations are less labor intensive compared with RNA libraries, where additional steps are required to convert RNA to complementary DNA for mNGS assays. Additionally, the natural instability of RNA makes nucleic acid extraction and library preparation more challenging for these assays, but commercial kits are available to aid in these applications. Automation is available for most library preparation workflows but is costly and does not necessarily result in time savings unless high volumes of testing are performed. A variety of NGS platforms are now available with sequencing times varying from several hours to several days. Sequencing time is not only dependent on the platform but also the number of samples included on the run and depth of sequencing required for the assay.
After the sequences for each sample have been generated, bioinformatics pipelines are utilized to apply defined criteria for acceptable sequence quality, eliminate human reads, and identify or match the sequences to a respective pathogen using open-source or curated databases. These pipelines can be either in-house developed, modified from open-source pipeline codes, or purchased from a number of commercially available companies, such as Taxonomer, OneCodex and CosmosID.5⇓-7 The development or implementation of published pipelines requires, at minimum, master’s-level training in computer science and bioinformatics with strong programming skills in Linux/Unix environments and common programming languages. Although commercial pipelines are easier to implement and use, some level of bioinformatics knowledge is ideal to aid in data manipulation, modification, and analysis. Taken together, implementation of mNGS requires development of new skills for most technologists and possible multidisciplinary team approaches with bioinformaticians and/or programmers who may not have training in clinical laboratory science. Importantly, there are currently no US Food and Drug Administration–approved approaches to mNGS for any element of the process, wet or dry bench. This means that adopting laboratories must devote significant financial and personnel resources to development, optimization, and validation of any approach.
ADVANTAGES OF mNGS AS A DIAGNOSTIC TEST
There are many advantages to mNGS over conventional cultures or serologic assays, with the main appeal being the ability to be completely unbiased. In addition to pathogen detection, mNGS also offers the opportunity to detect virulence determinants and resistance markers. The ability to sequence all nucleic acids present in the sample potentially allows for a more complete picture of the pathogen and may also allow incorporation of host biomarkers to help guide treatment and management decisions.
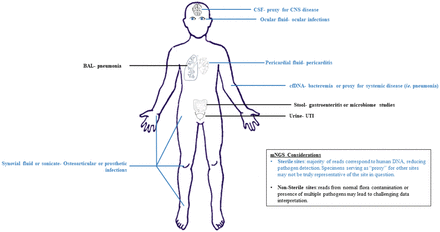
To date, most reports showing mNGS utility have been in cerebral spinal fluid (CSF) by both clinically available and research-use-only mNGS assays. However, many other specimens have been tested successfully, including research applications for other sterile sites (ocular and synovial fluid) and both research and commercial assays for plasma cell-free DNA (cfDNA) and nonsterile sites.
Sterile Sites
For sterile specimens directly from the infected source, a variety of pathogens have been detected. Metagenomic analysis of CSF for the diagnosis of central nervous system infections is perhaps the most high-profile application to date. The first real-time metagenomic diagnostic result was described in a child with presumed chronic bacterial meningitis of unknown etiology.8 mNGS yielded only 475 reads of Leptospira santarosai, which was later confirmed by serology and targeted PCR followed by Sanger sequencing. Since this initial report, additional cases or small case series have been published detecting novel or unexpected pathogens in CSF samples.3 Given the body of literature discussing mNGS applications for CSF, this will not be discussed in detail here and instead will focus on other sites where mNGS has applied.4,9 Osteoarticular infections (OAIs) are an attractive mNGS target because of the high proportion of culture-negative results, even in those cases wherein there is high suspicion for bacterial infection. OAI mNGS studies have tested a variety of specimens, such as periprosthetic tissue, synovial fluid, and explanted prosthetic joint sonication fluid. In one report, mNGS was applied to sonication fluid of an explanted knee arthroplasty to reveal infection was caused by Mycoplasma salivarium, which was confirmed by 16S Sanger sequencing.10 A larger study assessing mNGS for sonication fluids demonstrated 93% sensitivity and 88% specificity compared with culture.11 In this study, mNGS also potentially detected 12 additional bacterial infections not detected by culture, but these additional bacteria were of unclear clinical significance.
As with many diseases that are being targeted for diagnostic mNGS, ocular infections have a high proportion of cases with unknown etiology; more than 50% of ocular infections have negative findings by conventional testing.12 Infections of the eye are particularly challenging, as very limited volumes (100–300 µl) of ocular fluid can be safely obtained for testing.13 Additionally, infections caused by a virus, parasite, fungus, or bacteria may be clinically indistinguishable, making prioritization of testing by traditional methods difficult. Two recent studies by Doan et al applying DNA or RNA mNGS evaluated archived ocular fluid samples from patients with suspected or confirmed ocular infections. In the study, mNGS detected 27 of 31 pathogens identified by conventional testing.13 In almost a quarter of conventional test-negative cases (8 out of 36), mNGS identified viral pathogens (cytomegalovirus [CMV], human herpesvirus-6, herpes simplex virus [HSV]-2, and human T-lymphotropic virus-1), bacterial agents (Klebsiella pneumoniae), and yeast (Candida dubliniensis). An additional advantage of mNGS is the ability to provide resistance markers. In one report, mNGS provided sufficient coverage of the UL54 and UL97 genes of CMV to identify known mutations that correspond to resistance to ganciclovir and valganciclovir.13 In the RNA mNGS study, mNGS was able to correctly identify the infectious etiology for all 3 cases evaluated, including a parasite (Toxoplasma gondii), yeast (Cryptococcus neoformans), and virus (HSV-1). In an additional case of unknown etiology, mNGS resulted in the unexpected detection of Rubella virus, which was later confirmed with targeted PCR and Sanger sequencing. These studies highlight the tremendous value in the clinical application of unbiased mNGS, especially in low-volume critical samples.
Pericarditis, or inflammation of the membrane surrounding the heart, can be caused by noninfectious or infectious etiologies, and the ability to accurately distinguish is vital to patient management. Upward of 85% of pericarditis cases do not have an etiological agent identified.14,15 Molecular testing of pericardial fluid or tissue is usually required to make a final diagnosis. One study demonstrated the use of a metagenomics approach to identify Porphyromonas gingivalis from pericardial fluid, which did not grow in culture.16 Another study also used metagenomics to identify potential DNA viruses in patients with idiopathic pericarditis.17 Although still limited, these case reports clearly show the advantages of mNGS in diagnosing infectious diseases in which expansive traditional diagnostics are unrevealing or have been previously treated.
cfDNA
Similar to approaches used clinically for noninvasive fetal genetic testing, mNGS of cfDNA has also been explored. Application of mNGS to the diagnosis of sepsis is of obvious interest because of the wide breadth of organisms that can cause disease, particularly for the most at-risk patients. In a case report applying cfDNA mNGS of an asplenic septic patient, mNGS was able to detect Capnocytophaga canimorsus prior to blood cultures becoming positive. In this report, the patient had bacteria visible by Gram stain of blood, suggesting an extraordinarily high burden of bacteria, whereas only a small number of reads of C. canimorsus were detected.18 This highlights an important limitation to mNGS in which even in samples containing a high bacterial load, the sensitivity of mNGS is decreased due to amplification of all nucleic acid present in the sample. Although this is a select example of pathogen detection via mNGS, limited studies have addressed the clinical sensitivity and specificity of mNGS compared with conventional sepsis diagnostics. A recent paper assessed the analytical and clinical sensitivity of their laboratory-developed cfDNA mNGS assay for 350 patients presenting to the emergency department with sepsis found cfDNA mNGS was 84.9% sensitive and 62.7% specific when compared with traditional cultures,19 highlighting the fact that this approach is not necessarily more sensitive than current microbiologic diagnostics. Furthermore, the study produced an equal number of samples with probable pathogens compared with false positives identified by mNGS alone. In other words, when submitting a sample from a patient suspected of having sepsis, if a positive mNGS result is obtained, it may be equally likely to represent contamination vs a true pathogen.
In addition to detecting sepsis, cfDNA mNGS may also represent an approach to serve as a proxy for infection in a specific organ or elsewhere in the body (ie, use of cfDNA to detect infection of the lung). In this respect, cfDNA contains small nucleic acid fragments from dead organisms being filtered from other body sites for removal and clearance. The goal of using cfDNA mNGS as a proxy for remote sites would be to avoid invasive, high-risk procedures that are associated with increased morbidity and mortality, like a bronchoalveolar lavage (BAL) or biopsy. This same principle has been well described for noninvasive detection and treatment monitoring of organ malignancies.20,21 To date, a very limited number of studies using cfDNA in this manner have been published. One study attempted to use cfDNA to detect causative agents of invasive fungal infections (IFIs), which are a challenge to diagnose due to low yield in culture and lack of specific serum biomarkers.2 In this small study (n = 9), 7 out of 9 (77%) cases had the correct pathogen detected by cfDNA mNGS. However, some organisms that were detected by conventional culture were missed by mNGS, and mNGS detected some organisms that were missed by culture or of questionable significance. Larger studies are needed to assess the true sensitivity and specificity of this approach for IFI.
Nonsterile Sites
Application of mNGS for infectious disease in nonsterile sites are more complex and are only recently being explored. These sources present more difficulty with interpretation of mNGS data due to the presence of normal flora, an issue shared by culture. Similar to culture, quantitative mNGS approaches are likely the key to differentiating pathogens from commensal organisms. For example, the respiratory tract is normally colonized with microbial flora due to contact with the environment and, in deeper portions of the airway, possibly due to continuous microaspiration.22,23 In a recent study of upper respiratory tract samples from children with community-acquired pneumonia, mNGS was able to identify potential pathogens (Serratia marcescens and Pseudomonas fluorescens) that were not detected using traditional microbiologic methods. Additionally, these organisms were present in much higher proportion compared with other bacterial flora.24 A similar study investigating lower respiratory tract disease in BAL samples detected organisms not identified by conventional testing in almost half the cases.25 This included respiratory viruses (n = 4) and bacteria (n = 2; Streptococcus mitis and Corynebacterium propiniquum) that had at least 2-fold greater read proportions compared with other species in the same category (eg, “bacteria”); however, the significance of these findings are unknown, especially for the bacterial targets, which often represent normal flora. Quantitative analysis may also allow for better definitive identification of viral respiratory pathogens via mNGS. Significant positive correlation (90%) between normalized viral read counts by mNGS and viral load by quantitative polymerase chain reaction (PCR) have been shown.19,26⇓⇓⇓-30
Studies employing mNGS to the gastrointestinal (GI) tract have primarily focused on bacterial diversity, also known as the microbiome, which currently is more descriptive than actionable. However, limited studies have applied diagnostic mNGS in stool samples for detection of known GI pathogens. One study assessing an mNGS approach for the detection of Shigella and enteroinvasive Escherichia coli found that mNGS was accurate to detecting these pathogens but was no better than traditional culture.31 More studies are needed to fully understand the utility of mNGS from stool and other nonsterile sites.
mNGS applied to urine could allow for simultaneous detection of pathogens and antibiotic resistance genes or mutations, without the delay of culture and phenotypic susceptibility testing; however, microbiologic diagnosis of urinary tract infections relies on quantitative analysis of bacterial cultures because of the possibility of contamination during urine collection. Thus, mNGS may be overly sensitive for this application unless accurate cutoff criteria are established. A recent study applied shotgun mNGS to urine samples from symptomatic and asymptomatic individuals compared with routine culture.32 The authors proposed a cutoff based on total DNA quantity multiplied by the relative abundance of the dominant bacterial species detected by mNGS as a way to differentiate true infections from asymptomatic bacteriuria. This approach yielded ∼98% agreement with culture in the derivative dataset.
CHALLENGES, LIMITATIONS, AND OTHER CONSIDERATIONS OF mNGS APPLICATIONS
Although mNGS provides many advantages, there are significant technical and interpretative limitations that should be taken into consideration when developing, performing, or interpreting mNGS diagnostics. One main limitation is the sensitivity of truly unbiased mNGS, wherein, without a selection process, typically >99% of sequencing reads are of human origin.9,33 This decreases the sensitivity for pathogen detection. Selection steps that attempt to deplete human DNA or RNA in the sample prior to sequencing have been attempted, with varying increases in pathogen detection sensitivity.9,10,34 Although selection methods to specifically amplify or enrich for pathogen nucleic acid have been explored, these can reduce the unbiased nature of the mNGS by limiting the number of pathogens that can be detected. For example, an enrichment for pan-viral targets may be unbiased for viruses but excludes the potential to detect bacteria, fungi, and parasites. Additionally a larger or prospective clinical study, showing how often negative or difficult-to-interpret mNGS results are obtained and how often positive results are significant and/or clinically actionable, remains to be published.33
mNGS preparation is a multistep process, with many potential points for introducing contamination, including from the reagents used in the preparation, resulting in false positives.9,24,35 Often, results from mNGS can be difficult to interpret, especially if the organism detected is environmental, a part of the normal flora, or novel. For example, Fancello et al commonly detected 2 viruses, Anelloviridae and Retoviridae, in pericarditis patients included in the study. However, control patients with known noninfectious pericarditis also detected Retoviridae by this shotgun approach.17 Although the study shows the advantage of a highly sensitive metagenomics approach for pericardial fluid, it also highlights a major limitation, which is the clinical relevance and interpretation of the results, especially when novel or non–clinically relevant organisms are identified not known previously to cause disease. One group has chosen to ignore and not report such viruses when detected in CSF as a part of their clinical validation study.1 A similar limitation has also been shown for Rhinovirus and Bocavirus, which are commonly reported in respiratory tract mNGS studies.36 Although quantitative approaches are being explored, false-positive and difficult interpretations are likely for nonsterile sites where microbial reads more often represent normal flora instead of infection, a problem also shared by conventional methods. mNGS from plasma is particularly fraught with quality issues due to the low amount, and highly fragmented nature, of circulating nucleic acids. One study was able to detect a small number of reads (less than 1-fold coverage across the entire genome) of C. canimorsus DNA. This patient had bacteria consistent with C. canimorsus visible via staining of whole blood, suggesting his bacterial load was extremely high, which is likely the reason mNGS successfully detected the pathogen.18 Nevertheless, because of reported exposure history, this organism was high on the differential and was therefore empirically covered, questioning the translation of these results into clinical actions and modification in treatment decisions.33 cfDNA results may not always reveal the source of infection or true etiology due to high detectable microbial background; this has been shown in plasma cfDNA, which may confound results interpretation.19
If widespread adoption of mNGS is to occur, there will be a substantial shift in workforce and skillset needs. Because of the interdisciplinary approach of mNGS, the laboratory must expand to include expertise in clinical microbiology, infectious disease, molecular diagnostics, computer programming, and bioinformatics. Laboratory staff will need training in high-complexity library preparation, instrument function, maintenance, and troubleshooting that is specific to the NGS platform(s) and application(s) that are to be used. An in-depth understanding of the technology and the purpose of each step in the mNGS process and critical thinking skills will be needed to aid in assay optimization and problem solving. Additionally, a portion of the laboratory workforce will need to have higher degrees or senior experience in bioinformatics to conduct analysis and troubleshooting of mNGS sequencing data, including pipeline development or pipeline modifications to meet the needs of the diagnostic assay being performed (Table 1).
Sites of current and future mNGS diagnostics. Blue represents sterile sites. Black represents nonsterile sites. BAL, bronchoalveolar lavage; cfDNA, cell-free DNA; CNS, central nervous system; CSF, cerebral spinal fluid; UTI, urinary tract infection.
mNGS steps and needed skillsets
THE FUTURE OF mNGS
As we continue to learn more about how to perform, quality control, and interpret mNGS diagnostics, this method will likely become more standardized in large clinical laboratories. However, it is unlikely to replace traditional cultures and other molecular diagnostics completely. Although mNGS appears to provide no advantage in the detection of routine and commonly detected pathogens, its utility appears to be best when applied to cases where traditional diagnostics are negative or when atypical pathogens are suspected. Additionally, there may be an advantage to mNGS assays for the critically ill to provide more rapid, all-encompassing results to impact management decisions. The enthusiastic interest in developing mNGS diagnostics will continue to evolve and improve this methodology and, over time, will find its rightful place among our clinical microbiology toolkit for the detection of infectious agents.
- Received June 3, 2019.
- Accepted September 5, 2019.
American Society for Clinical Laboratory Science