This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- California State University, Dominguez Hills
- California State University, Dominguez Hills
- California State University, Dominguez Hills
- Address for Correspondence: Payman Nasr
, California State University, Dominguez Hills, pnasr{at}csudh.edu
ABSTRACT
Apart from severe acute respiratory syndrome and cytokine dysregulation, novel coronavirus disease 2019 (COVID-19) infection has also been associated with a broad range of complications including septicemia, thrombosis, thrombocytopenia, Guillain–Barre, antiphospholipid syndrome, and multiple organ failure. Warm autoimmune hemolytic anemia (WAIHA) in its clinical presentation is similar to other forms of hemolytic anemias in which hemoglobin level is the most direct indicator of clinical severity of the hemolytic event. In this report, we describe the case of a 31-year-old male, previously diagnosed with idiopathic WAIHA, who goes on to suffer a severe flare following a COVID-19 infection. Prior to the COVID-19 infection, the patient was in a stable condition and was taking part in a clinical study for the treatment of WAIHA. In the case of this patient, the COVID-19 infection elicited a severe hemolytic episode, which required admission to the hospital and multiple units of blood transfusion. The patient’s hematological recovery correlated with improvement from COVID-19 symptoms. The patient was stabilized with glucocorticoids and discharged after 2 weeks of hospitalization. The current case supports the role of COVID-19 infection as a comorbidity factor in WAIHA.
- ACE-2 - angiotensin-converting enzyme
- AIHA - autoimmune hemolytic anemia
- CoV - coronavirus
- COVID-19 - coronavirus disease 2019
- LDH - lactate dehydrogenase
- MERS - Middle East respiratory syndrome
- RBC - red blood cell
- SARS - severe acute respiratory syndrome
- SARS-CoV-2 - severe acute respiratory syndrome coronavirus 2
- WAIHA - warm autoimmune hemolytic anemia
- WBC - white blood cell
CASE DESCRIPTION
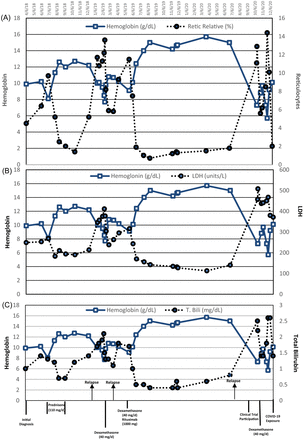
A 31-year-old Hispanic male sought medical attention after daily headaches, paresthesia, dyspnea, chest pain, and mild cognitive impairment. The patient reported no history of blood transfusion in the past, no history of acute infections or chronic inflammatory conditions. The initial physical exam showed a general pallor paleness of the skin, blood pressure 140/100 mmHg, with fast but strong heartbeats (95 beats/min), and palpable spleen on deep inspiration. The transverse and longitudinal ultrasound showed mild hepatosplenomegaly with hepatic steatosis, and the spleen was palpable on the left upper abdomen on deep inspiration. The first laboratory results were remarkable for erythrocytopenia: low hemoglobin and hematocrit and an increase in reticulocyte percentage, total bilirubin, and sedimentation rate. Lactate dehydrogenase (LDH) was slightly elevated (238 U/L; reference 135–225 U/L). Haptoglobin concentration was below the instrument detection limit (<15 mg/dL). The liver profile was unremarkable, and the acute hepatitis panel was nonreactive. Iron studies included the percent transferrin saturation rate and the total iron-binding capacity within the reference intervals, whereas ferritin was elevated (500 ug/L; reference 26–336 ug/L). Direct antiglobulin test was positive for anti-IgG and anti-C3. Clinical assessments excluded mineral and vitamin deficiencies, lymphoproliferative disorders, nonlymphoid neoplasms, rheumatoid arthritis, systemic lupus erythematosus, and immunodeficiency disorders and, ultimately, yielded the diagnosis of idiopathic IgG plus complement warm autoimmune hemolytic anemia (WAIHA). Refer to Figure 1 to view the progression of the disease and the associated treatment interventions.
The chronological comparison of the laboratory results for the relative reticulocyte percentage, lactate dehydrogenase (LDH), and total bilirubin (T. Bili), with respect to the hemoglobin values since the onset of the first hemolytic episode. (A) Hemoglobin versus relative reticulocyte percentage, (B) hemoglobin versus LDH, (C) hemoglobin versus total bilirubin. The arrows indicate relapses, whereas the straight vertical lines show each treatment regimen.
Attaining and maintaining remission proved challenging. Following diagnosis, prednisone (110 mg/day) was administered for 2 months with taper. Laboratory parameters normalized in 1 month, but after a 3-month remission, symptoms recurred characterized by a rapid drop in hemoglobin. As an alternative therapy, the patient received pulse dexamethasone 40 mg daily for 4 days, with alleviation of symptoms. A 3-month remission was followed by another relapse. A new treatment approach with dexamethasone and rituximab, a monoclonal antibody targeting CD20-positive B lymphocytes (2 doses of 1000 mg of rituximab with 40 mg dexamethasone for 4 days), was administered, improving the physical symptoms and normalizing the laboratory parameters within 2 weeks. The patient remained in remission for 16 months before the recurrence of the hemolysis and anemia symptoms, at which time he joined a multicenter, randomized, double-blind, placebo-controlled study of fostamatinib disodium hexahydrate for the treatment of WAIHA. Following the treatment with 40 mg dexamethasone for 4 days, hemoglobin value was stabilized at 10 mg/dL with the resumption of daily activities. Figure 1A–C shows patient’s percent reticulocytes, LDH, and total bilirubin with respect to the hemoglobin concentration from the initial diagnosis to the coronavirus disease 2019 (COVID-19) exposure.
On December 7, 2020, the subject’s father, who lived in the same household, was notified of COVID-19 exposure at his workplace. Same-day COVID-19 testing was negative for every member of the household. The next testing on December 10, however, showed a positive result for the father. Every member of the household, including the case subject, tested positive 1 day later on December 11. On December 14, 3 days following the positive COVID-19 result, although the case subject was physically asymptomatic for COIVD-19, the laboratory results showed a general pancytopenia (white blood cell [WBC] count 1.52 × 109/L, red blood cell [RBC] count 3.15 × 1012/L, platelets 121 × 109/L) and early signs of hemolysis (hemoglobin 10.1 mg/dL, LDH 371 U/L, haptoglobin <15 mg/dL, total bilirubin 2.0 mg/dL). On December 19, the patient reported the symptoms of a hemolytic episode (eg, severe fatigue, fever, dark urine color), which led to his hospitalization. The hemoglobin value on the date of hospital admission was 6.8 mg/dL, whereas unlike the previous hemolytic episodes, the D-dimer (>20 ug/ml; reference <0.6 ug/ml) and the fibrinogen (505 mg/dL; reference 235–490 mg/dL) were elevated. The patient was initiated on a regimen of steroids (dexamethasone 40 mg/daily for 4 days) and prophylactic anticoagulant therapy (enoxaparin sodium 40 mg subcutaneous) and monitored closely for deep vein thrombosis and pulmonary embolism. The continuous drop in hemoglobin prompted blood transfusion. On December 20, the first unit of blood was transfused, which resulted in a transfusion reaction and a severe hemolytic episode with a subsequent hemoglobin of 3.9 mg/dL. The patient received 7 additional units of irradiated and washed RBCs at a slow rate of infusion over the next 9 days. Table 1 shows the values for hemoglobin, total bilirubin, fibrinogen, and D-dimer and the treatment notes from the hospital admission to discharge.
CASE RESOLUTION
The patient’s hematological recovery trended with improvement from COVID-19 symptoms. As the COVID-19 symptoms were resolved, the reticulocyte values recovered (19.2% on 12/30/2020). The patient was kept on a treatment with 110 mg daily prednisone until the hemoglobin values increased to 8.1 mg/dL, at which time the patient was discharged taking 50 mg of prednisone a day, with taper.
DISCUSSION
Since the World Health Organization declared COVID-19, which is principally a respiratory infection, a pandemic on March 11, 2020, clinicians have been reporting extrapulmonary complications.1⇓⇓-4 Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been associated with cardiac, neurologic, dermatological, renal, and gastroenterological manifestations and septicemia, Guillain–Barre, antiphospholipid syndrome, and multiple organ failure.5⇓⇓-8 Hematologic abnormalities have also been reported following COVID-19 infection, with alterations seen in platelets, RBCs, hemoglobin, lymphocyte, granulocyte, monocyte, macrophage, and coagulation responses.9,10 SARS-CoV-2 has also been implicated in immune dysregulation and the development of autoimmune diseases.10⇓⇓-13 The relationship between viral infections and the potential development of autoimmune diseases is widely acknowledged. A number of hypotheses have been proposed to explain how and why this occurs, with molecular mimicry or crossreactivity cited in many cases.2,14,15 Along with severe acute respiratory syndrome (SARS)-coronavirus (CoV) and Middle East respiratory syndrome (MERS)-CoV, SARS-CoV-2 is classified as a β-CoV and is commonly associated with severe respiratory syndrome.16⇓-18 It has been reported that SARS-CoV-2 utilizes angiotensin-converting enzyme (ACE-2) receptor to penetrate and infect the cells similarly to mechanisms seen in other SARS-CoV infections.19,20 CoVs have 4 structural proteins: spike (S), membrane (M), envelope, (E), and nucleocapsid (N).21 The proposed model for viral fusion requires a 2-step protease cleavage to activate the spike protein, which has been identified to be used by SARS-CoV and MERS-CoV to enter the cells after binding ACE-2. However, SARS-CoV-2 also utilizes protease furin, which other CoVs have not shown to use and may account for increased SARS-CoV-2 pathogenicity.3 It is speculated that the presence of redundant furin cut sites in the spike protein is responsible for SARS-CoV-2’s higher membrane fusion efficiency and enhanced pathogenicity compared with other CoVs.22 ACE-2 and furin are coexpressed membrane endopeptidase of the ACE-2 receptor, and furin has the potential to cleave the viral envelop glycoprotein, thereby enhancing the viral fusion with host cell membranes.23⇓-25
Although hematologic autoimmune cases, including autoimmune hemolytic anemia (AIHA), linked to COVID-19 infection, are slowly being reported,26,27 the impact of COVID-19 on preexisting AIHA has only been documented in isolated cases. The current literature has primarily focused on patients who have developed AIHA during or post–COVID-19 infection. Lazarian et al described 7 patients who developed AIHA after testing positive for COVID-19.27 Lopez et al reported a case of simultaneous presentation of COVID-19 and WAIHA.26 Hindilerden et al presented a case of a patient with moderate COVID-19 pneumonia who went on to develop AIHA,28 whereas Capes et al described a patient in the intensive care unit suffering from COVID-19 infection who, at around 2 weeks after admission, began showing signs and symptoms of AIHA.29 Most recently, Finkenthal et al published a case of a patient with a history of well-controlled AIHA secondary to systemic lupus erythematosus whose AIHA was exacerbated by COVID-19 infection.30 WAIHA is the most common form of AIHA and is characterized by binding of panreactive polyclonal IgG to Rh proteins or glycophorins on the RBC surface optimally at 37 °C, targeting the cells for premature removal by macrophages in the spleen and other extravascular sites.31 The clinical presentation of WAIHA ranges from general symptoms of anemia (fatigue, dyspnea, tachycardia) in mild cases, to fever, jaundice, hepatosplenomegaly, and renal failure in the more severe cases.32 Corticosteroid therapy is the standard initial treatment for moderate to severe cases of WAIHA, whereas in recent years, a combination therapy with rituximab, a chimeric, mouse/human, monoclonal antibody targeting CD20, has shown promising results.33 In the current case, following the rituximab with dexamethasone pulse therapy, the laboratory parameters normalized within 2 weeks, and the hemoglobin level achieved the highest level since the first onset of symptoms, a goal that was not reached with glucocorticoid monotherapy. The patient remained in remission for 16 months before relapse, at which time the patient joined a double-blind clinical trial for fostamatinib disodium hexahydrate, a tyrosine kinase inhibitor. The subject had to withdraw from the clinical study during a severe hemolytic crisis following COVID-19 infection. Three days after a positive COVID-19 test result, although the subject was asymptomatic for COVID-19, the laboratory results showed elevated hemolysis marker and pancytopenia. The subject was hospitalized 8 days after the initial positive COVID-19 test results with mild respiratory symptoms and a severe hemolytic episode that was exacerbated by the blood transfusion reaction, which highlights the therapeutic application of irradiated blood at a low infusion rate in patients with autoimmune disorders (see Table 1).
Hemoglobin, total bilirubin, and treatment notes
Following the hospital admission, the initial laboratory results showed an increase in D-dimer and a mild elevation in fibrinogen, indicating a hypercoagulability state. Previous reports have shown that COVID-19 infection may result in elevated coagulation levels and poor prognosis.34⇓-36 However, in the current case, considering the application of prophylactic anticoagulant therapy, the patient’s results did not meet the criteria set by the International Society on Thrombosis and Haemostasis for Disseminated Intravascular Coagulation Score (<5).37⇓-39
Although it is difficult to determine the exact dynamics of the hemolytic crisis, immunosuppressive treatments, and COVID-19 infection, the current case suggests that COVID-19 infection exacerbated the hemolytic episode by inducing pancytopenia as detected by decreased reticulocytes, WBCs, and platelets before the onset of the COVID-19 symptoms. The case outcomes also suggest that the standard treatment of AIHA with corticosteroids is unaffected by the COVID-19 pandemic, but the addition of COVID-19 may worsen the hemolytic episode and require hospitalization and multiple-unit blood transfusion. The current case describes a patient with WAIHA who goes on to experience a severe flare and hemolytic episode after COVID-19 infection. This report aims to contribute to the small but growing body of evidence that examines the risks associated with COVID-19 infection in patients who have previously been diagnosed with WAIHA.
FUNDING
The American Society of Clinical Pathologists Laboratory Science Program Director Educational Grant, 2021.
ACKNOWLEDGEMENTS
The authors wish to thank Professor Cheryl Jackson-Harris for her invaluable input in the case assessment.
- Received March 15, 2024.
- Accepted June 6, 2024.
American Society for Clinical Laboratory Science