This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Curtiss V Johnson
, Mercy St. Vincent Medical Center, johnsonc325{at}yahoo.com
ABSTRACT
Serum protein electrophoresis (SPE) is a valuable tool for clinical laboratories to identify various protein abnormalities and disease states. Given certain SPE patterns, it is beneficial to know if subsequent immunofixation electrophoresis (IFE) is warranted to detect underlying abnormal bands and whether further clinical correlation is recommended. Using a sample of 160 patients in 1 hospital lab, it was possible to involve both medical technologists and clinical pathologists in identifying abnormal SPE patterns of interest and to craft recommendations for using follow-up IFE to detect clinically significant protein abnormalities. Utilizing a similar process and guidelines of this nature may be beneficial in increasing diagnostic efficiency and efficacy for clinical labs performing electrophoretic testing on patients presenting with such patterns.
INTRODUCTION
Serum protein electrophoresis (SPE) is a common method for diagnosing a variety of abnormalities and diseases related to human protein levels and composition. These frequently include instances of acute and chronic inflammation, nephrotic and liver disorders, monoclonal gammopathies (including multiple myeloma), and hereditary disorders.1 In some abnormal SPE patterns, immunofixation electrophoresis (IFE) is utilized as a follow-up methodology to further identify and quantitate abnormal zones of restriction. This is primarily used to classify monoclonal immunoglobulins in serum but also has applications in diagnosing light chain diseases in urine (namely Bence Jones proteinuria) and detecting more subtle bands outside of the gamma globulin region that can be of clinical significance.2,3
This article serves to highlight select groups of SPE patterns that may present challenges in interpretation and offer suggestions for performing medical technologists to assess whether a follow-up immunofixation is indicated. By utilizing the laboratory expertise of a medical technologist, in consultation with a clinical pathologist, performing laboratories may benefit from improved efficiency and accuracy that come from proper use of IFE in these cases.
MATERIALS AND METHODS
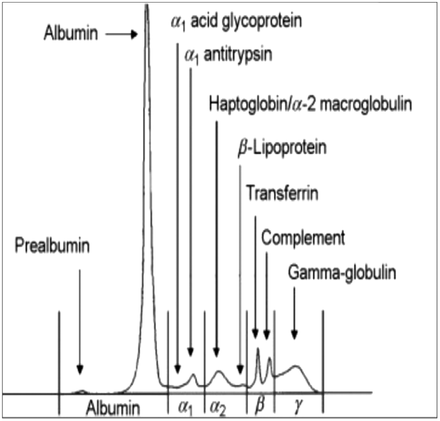
All patient testing and interpretation of SPE patterns were performed at Mercy Integrated Laboratories in Toledo, OH by American Society for Clinical Pathology board-certified medical laboratory scientists and clinical pathologists. Testing was performed utilizing the Sebia Hydrasys 2 Scan instrument as directed in approved laboratory policy and procedure manuals. Reference ranges for albumin, alpha-1, alpha-2, beta, and gamma fractions were set by the performing laboratory and according to clinical guidelines (a normal SPE pattern4 is shown for reference in Figure 1).
Normal serum protein electrophoresis pattern 4. Reprinted from Advances in Clinical Chemistry, volume 42, Xavier Bossuyt, “Advances in serum protein electrophoresis,” pages 43–80, copyright (2006), with permission from Elsevier.
A total of 160 patient samples were collected over a 3-month period and reviewed for the following patterns of interest: acute phase reaction (including C-reactive protein [CRP]), fibrinogen bands, beta zone abnormalities, polyclonal hypergammaglobulinemia, and hypogammaglobulinemia. Following testing, a medical technologist reviewed and correlated results from patients exhibiting these patterns with published literature and a consulting clinical pathologist to assess overall clinical significance and whether or not a subsequent immunofixation was indicated for each categorized group of SPE patterns. Recommendations regarding the diagnostic utility of IFE in detecting underlying abnormalities in these patterns were crafted based on established literature and the following results (summarized in Table 1).
IFE results for selected SPE patterns of interest
RESULTS
Of the 160 samples collected, 70 were classified as normal electrophoretic patterns. Twenty-seven of these normal samples had a subsequent IFE performed and were negative for monoclonal immunoglobulin. Given the normal initial SPE patterns, these samples were not included in the review and analysis for the purposes of this study.
Twenty-one samples exhibited patterns suggestive of an acute phase response (decreased albumin and/or increased alpha-1, alpha-2). Of these acute phase-like patterns, 13 patients had an IFE performed. Eleven were negative for monoclonal immunoglobulins, and 2 were positive for diclonal IgG lambda and free monoclonal lambda chains, respectively. Seven patients demonstrated a true characteristic acute phase response of decreased albumin and increased alpha-1 and alpha-2 globulins. One of these patterns showed an elevated level of CRP in addition to a fibrinogen band. Subsequent immunofixation for this patient was negative.
Four samples demonstrated an apparent beta zone abnormality, each of which had a positive follow-up IFE. Two patterns were confirmed as diclonal IgA lambda, with the remaining patterns confirmed as monoclonal IgG kappa and IgA kappa, respectively.
Fourteen samples exhibited either a borderline or true polyclonal hypergammaglobulinemia. Eight of these had an IFE performed, 3 of which were positive, and 5 of which were negative. Positive samples respectively demonstrated zones of restriction including diclonal IgG lambda, monoclonal IgG kappa, and monoclonal IgG lambda. The remaining patterns in this categorized group did not have an IFE performed.
Seventeen samples displayed mild or pronounced hypogammaglobulinemia, and IFE was performed on each patient in this group. Thirteen of these exhibited a positive immunofixation, with differing zones of restriction in the gamma globulin region for each patient. Each zone of restriction was confirmed to be either monoclonal immunoglobulin (most commonly IgG kappa or lambda) or monoclonal light chains (free kappa or lambda). The remaining 4 patterns were negative for monoclonal immunoglobulins of any kind.
DISCUSSION
Given the IFE results for these SPE patterns of interest, with consideration of previously established literature on these topics, we have crafted the following summary, guidelines, and recommendations for follow-up as shown in Table 2.
Summary of recommendations for follow-up of select SPE patterns
The acute phase reaction is a typical representation of an inflammatory immune response. While the cause of such responses can include infections, trauma, burns, and tissue necrosis, these often manifest as an easily identified electrophoretic pattern of lowered albumin levels and elevated alpha-1 and alpha-2 globulins.5 During the late stages of an acute phase reaction, one may observe elevated levels of CRP, which often appears as a faint band in the midgamma region. This may erroneously appear as a small monoclonal gammopathy, but by correlating SPE manifestations (stated above) of an acute phase reaction along with the patient’s clinical presentation and other laboratory values, performing technologists can accurately identify this phenomenon and report these patterns accordingly.6 Given the predictable nature of acute phase reactions and small percentage of patients in this study warranting follow-up IFE, immunofixation is rarely indicated for these patterns.
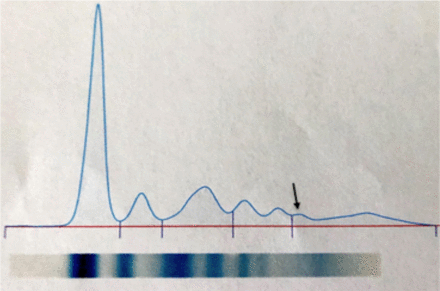
One instance that warrants performing IFE is the presence of a clear fibrinogen band in an initial SPE pattern. Appearing in the fast gamma region, this is commonly an artifact that arises due to insufficient plasma clotting before analysis or in patients receiving heparin therapy.7 While typically alleviated by treating the sample with 10% ethanol or thrombin prior to analysis, fibrinogen has been known to mask or interfere with the detection of clinically significant bands in the gamma globulin region. Thus, further clinical correlation with a patient’s medication list and coagulation studies may be needed to associate fibrinogen as the cause of an abnormal zone of restriction in the gamma region.8 Despite the single patient in this study presenting with a clear fibrinogen band (Figure 2) having a negative subsequent IFE, any cases of this nature should generally be followed up with immunofixation to rule out an underlying monoclonal gammopathy or other potentially hidden bands in the gamma globulin region.
Arrow indicates a clear fibrinogen band present in this patient sample.
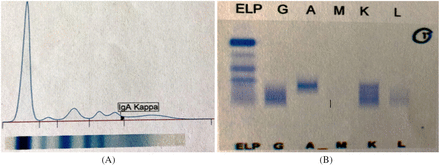
Similarly, any abnormalities involving the beta globulin region should warrant IFE. The beta fraction comprises varying components, including transferrin, beta-lipoprotein, and complement proteins. While spikes in this region have clinical correlations ranging from iron-deficiency anemia to nephrotic and thyroid disorders, monoclonal immunoglobulins including IgA, IgM, and IgG have been known to appear1,5 (Figure 3A). Occasionally, the appearance of these may be implicated in patients with multiple myeloma. While the characteristic M-protein (most commonly IgG) typically migrates to the gamma region, there are documented instances in which an IgA variant of this protein migrates to the beta region (Figure 3B), making it difficult to interpret this region and quantitate the abnormal monoclonal protein.9,10 Considering that each patient in this study exhibiting beta zone abnormalities had a positive follow-up IFE, additional laboratory testing and correlation with patient history and symptoms may be required to rule out an atypical presentation of multiple myeloma or other monoclonal gammopathies. Nevertheless, immunofixation is recommended when presented with abnormal SPE patterns of this nature.
(A) Initial SPE pattern with apparent beta zone abnormality. (B) Follow-up IFE identifying band as IgA kappa. Abbreviations: IFE, immunofixation electrophoresis; SPE, serum protein electrophoresis.
Patterns exhibiting polyclonal hypergammaglobulinemia may require follow-up immunofixation. In contrast to the distinctly sharp bands present in monoclonal gammopathies, polyclonal hypergammaglobulinemia presents as a more broad, diffuse band in the gamma region. This often occurs during nonmalignant conditions such as chronic inflammation, infections, and various liver or autoimmune diseases, so IFE is typically not required in cases wherein correlation with patient symptoms and other lab tests (such as complete blood count or metabolic panel results) can identify the underlying condition causing a polyclonal band on initial SPE.5,11 However, immunofixation remains important in differentiating from more severe monoclonal gammopathies and other malignancies. Thus, IFE may be helpful in cases wherein questionable bands are present or if the underlying cause of polyclonal hypergammaglobulinemia is not clearly correlated with patient’s medical history, lab results, and clinical presentation. Considering the heterogeneity of IFE tests performed and results for patients in this category, it is best to consult with patient’s providers and a clinical pathologist for such cases.
Cases of hypogammaglobulinemia nearly always warrant immunofixation due to the potential for atypically presenting malignancies. In some instances, IFE may reveal low-concentration M-proteins or other monoclonal spikes in an otherwise normal SPE pattern. For example, a small percentage of patients with multiple myeloma exhibit hypogammaglobulinemia without the characteristic M-protein spike on SPE, so interpreting such patterns is complicated by the inability to quantitate the malignant paraprotein. These patients often have increased levels of free monoclonal kappa or lambda light chains (Bence Jones protein) present in urine, so correlation with urine SPE/IFE patterns along with urinalysis and other lab values is recommended to gain a full clinical picture in these cases.1,12 An elevated level of alpha-2 globulin and alpha-2 to alpha-1 ratio also has moderate predictive value for a positive IFE in normal SPE patterns, so performing technologists should be aware of these and other SPE findings that may require follow-up IFE in patients exhibiting hypogammaglobulinemia.13
In some cases of hypogammaglobulinemia, IFE can also help identify subtle, treatment-related bands in individuals with a previously documented paraprotein in the gamma globulin region. For example, in patients with known IgG kappa myeloma, certain medications (daratumumab, elotuzumab, etc) are known interferents in SPE testing due to the similar migratory patterns of these therapeutic monoclonal antibodies.12 For individuals receiving such treatments, it is important to correlate these findings with the patient’s clinical condition and medication list in order to account for any suspicious monoclonal bands and limit unnecessary follow-up.
Due to the prevailing evidence regarding the need for immunofixation in patients demonstrating hypogammaglobulinemia, each patient in this group had a subsequent IFE performed. As indicated by the high percentage of positive immunofixations in this group, the clinical utility of IFE in such cases is clearly substantiated, and further clinical investigation is often warranted for patterns of this nature.
CONCLUSION
The patterns selected for this study represent just a small sample of the diagnostic applications of SPE and IFE methodologies. Nevertheless, it is important for clinicians to recognize these patterns to discover potentially significant alterations in protein levels and composition. While subsequent immunofixation is not always required, SPE and IFE remain valuable tools for detecting abnormalities within these selected electrophoretic patterns and should be utilized accordingly.
Given these results and knowledge gained from published medical literature, medical technologists can be trained to recognize these SPE patterns and take appropriate action to characterize abnormalities in consultation with a clinical pathologist. Involving the performing medical technologist and pathologist in these decisions can help better manage time and resources for both patients and providers, improving the diagnostic efficiency and effectiveness of SPE testing for the performing laboratory and their patient population. Future consideration should be given to crafting similar guidelines of this nature for clinical laboratories wishing to maximize the utility of SPE and IFE testing methods.
ACKNOWLEDGEMENTS
We thank the Department of Chemistry at Mercy Integrated Laboratories for their role in SPE/IFE test performance and data collection.
- Received March 2, 2024.
- Accepted August 11, 2024.
American Society for Clinical Laboratory Science