This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Rodney E. Rohde
, Texas State University, rrohde{at}txstate.edu
LEARNING OBJECTIVES:
1. Explain historical and current examples of how select pathogens can evade antimicrobial treatments and how this confers an evolutionary advantage to that pathogen.
2. List and describe the effects of globalization in the spread of antimicrobial-resistant (AMR) pathogens, particularly in international travel and urbanization.
3. Describe how proper public policy, medical intervention strategies, and development of novel therapies can all be used to curtail the emergence of AMR pathogens.
ABSTRACT
Antimicrobial resistance (AMR) is a complex issue that has currently reached a dangerous tipping point. The nature of this critical public health and healthcare problem has two primary components: (1) the emergence of diverse AMR pathogens and (2) the alarming rapid spread of these AMR pathogens in a wide array of geographic and densely populated regions. The adaptation and survival of these dangerous pathogens are associated with ongoing and increasing natural selective pressure as it relates to human, animal, and environmental health settings. The primary drivers for this mounting trend of AMRs include but are not limited to changing patterns of pathogen epidemiology, emergence of drug-resistance genes, animal husbandry, antimicrobial use/stewardship, population mobility, increased rates of human urbanization, and the movement and ease of products and goods across global settings. Simply stated, AMR pathogens are incredibly versatile biological entities at adaptation to every natural (and unnatural) niche known and make themselves at home regardless of where they land. Understanding AMRs is critical to curbing their ongoing global drift. For this special article, we will address the emergence and spread of AMR pathogens from a medical laboratory and public health lens and how this new threat affects the ever-evolving relationship between hosts and pathogens.
- AMR - antimicrobial resistance
- HAI - healthcare-associated infection
- HIV - human immunodeficiency virus
- MRSA - methicillin-resistant Staphylococcus aureus
- Mtb - Mycobacterium tuberculosis
- PBP2A - penicillin-binding protein 2A
- WWII - World War II
- antibiotic resistance
- antimicrobial resistance
- globalization
- healthcare-associated infections
- population mobility
- urbanization
INTRODUCTION
Antimicrobials have played a pivotal role in public health. The majority of their mechanism of actions is to target a pathogen-specific process, such as the synthesis of the bacterial cell walls in bacteria (eg, streptococci) or the reverse transcriptase used by viruses (eg, human immunodeficiency virus [HIV]). There are, of course, exceptions to this, but for this manuscript, we will focus on pathogen-specific processes. Although these antimicrobials initially showed remarkable effectiveness, many of their effects are waning. Healthcare-associated infections (HAIs) caused by pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), cost the healthcare industry billions each year because of their difficulty to treat. As one can surmise by the name, the difficulty in treating these pathogens is attributable to their antimicrobial resistance (AMR). The same drugs that were once used to treat such infections have lost much of their utility, allowing for the spread of AMR pathogens.
Although frequently associated with antibiotics, AMR is not a phenomenon exclusive to bacteria. Other pathogens, including viruses1⇓⇓⇓⇓⇓-7 and protozoa,8⇓-10 also exhibit this ability to respond to antimicrobials, further demonstrating the dangerous scope of AMR in infectious diseases. Therefore, AMR represents a massive public health concern for pathogens ranging from HIV to Plasmodium falciparum (the malaria parasite). This puts a tremendous burden on healthcare providers and healthcare infrastructure, particularly in developing countries where resources can already be scarce.11
A number of factors are involved in the spread of AMR pathogens, which we will touch upon in this manuscript. It is not possible to address them all in their entirety nor in the detail this topic deserves, but we will address the foundational drivers of this public health crisis. From the acquisition of AMR genes into the pathogen population to the increased population condensation into urban areas, AMR pathogens have been able to establish themselves into our current society.12,13
THE PENDULUM OF AMR
The use of antibiotics, an antimicrobial agent targeted toward bacteria, burst into prominence during World War II (WWII). Wound sepsis was a leading cause of death and disablement among military personnel, and the need to develop rapid therapeutics against infections was needed as the world entered into conflict. Hence, a large amount of attention into the recent discovery of a novel antibacterial agent, penicillin, was paid. Its discovery and initial publication in the late 1920s were perhaps the most consequential and fortuitous observations in medicine of that century. The antibiotic was discovered by Dr Alexander Fleming when he noticed that his Staphylococcus cultures were destroyed by a cocontaminating mold, Penicillium notatum. Upon purification of the compound responsible for the antimicrobial behavior, physicians and military personnel realized its potential use to treat battlefield injuries and the US and British governments invested heavily in the bulk production of the antibiotic. Indeed, penicillin was broadly utilized by allied troops throughout WWII and saved many thousands of lives. In hindsight, the broad and medically unsupervised use of penicillin in the war theater was an early and then unknown push of the lever toward AMR. This problem has not yet been resolved and has actually gotten much worse.
Penicillin acts by inhibiting cell wall synthesis of bacteria, thus explaining how the mold was able to kill Dr Fleming’s Staphylococcus cultures. The molecule directly targets the peptidoglycan synthesis, a saccharide-rich component of the cell wall of gram-positive and, to a lesser extent, gram-negative bacteria. Penicillin directly binds to and inhibits bacterially encoded enzymes involved in the peptidoglycan synthesis (termed transpeptidases), thus destabilizing the integrity of the bacterial cell. Since its discovery by Dr Fleming, a number of penicillin-derivative antimicrobials have been developed, including ampicillin, amoxicillin, methicillin, etc. Given their target, these drugs collectively fall into a broader family of antimicrobials called the β-lactam antibiotics.11,14,15
ADAPTATION OF BACTERIA TO THE BATTLEFIELD
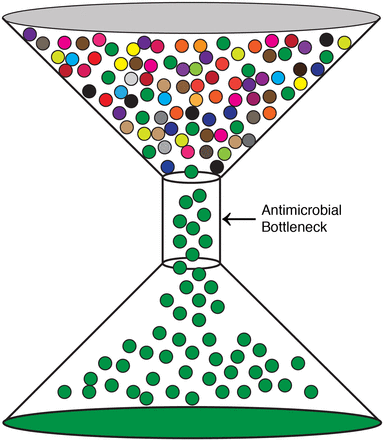
Given their propensity for elimination of pathogens, antimicrobials, such as the β-lactams, have been widely prescribed for bacterial infections. Unfortunately, this selective pressure has given rise to AMR pathogens. This can be visually articulated in terms of the bottleneck effect (Figure 1). A selective pressure, in this case, an antimicrobial, is applied to a population, in this case, a pathogen. This will result in the death of the majority of the population, but a small fraction of the input population will escape this pressure and multiply. This escaping population of the pathogen is more equipped to then survive subsequent selection pressures by the same antimicrobial. This is a basic principle of selection described by Charles Darwin: Variations within a population that are desirable will proliferate, whereas undesirable traits will be progressively eliminated.16 We have been imposing selective pressures on pathogens via antimicrobials for decades, and AMR pathogens have become increasingly more prevalent as a result.
The bottleneck effect. A population of a pathogen is exposed to an antimicrobial. The majority of the pathogen population contains no resistance, but a fraction does (green) and can flow through the bottleneck. This population can then propagate, leading to the rise of AMR in that particular pathogen.
The rise of MRSA is an excellent example of how AMR pathogens can wreak havoc on our medical infrastructure and public health. MRSA is a strain of S. aureus that has developed a strong resistance to the prescribed β-lactam antibiotic methicillin. This AMR is conferred by the gene mecA, which encodes for the protein penicillin-binding protein 2A (PBP2A),14,15,17,18 a transpeptidase involved in peptidoglycan synthesis. Unlike other transpeptidases, the β-lactam antibiotic does not directly bind to PBP2A, allowing the enzyme to synthesize the cell wall and preserve the integrity of the bacterial cell. Like other staphylococci, MRSA encodes for a number of virulence factors that can cause local tissue damage and even lead to toxic shock syndrome.11,19⇓⇓-22 Therefore the presence of PBP2A is a potent driver of pathogenesis as it confers an advantage to the bacteria.
When it comes to harnessing beneficial genes, AMR pathogens are truly a model organism for microbiologists to study. Bacteria can acquire and subsequently transfer resistance genes through several methods. One mode of gene acquisition is through a process termed conjugation. Conjugation is a rapid mechanism in which bacteria cojoin an outer appendage (pilus) to copy over different genes (traits) from extrachromosomal plasmid DNA-like toxin and resistance genes. This has been observed for genes, such as mecA, in which the gene can be rapidly transferred horizontally to other genera and species of bacteria.23,24 If one envisions the healthcare environment rich with HAIs and AMR pathogens, this scenario becomes even more problematic in the mingling of AMR pathogens with “regular bacteria” because of this horizontal transfer issue. The transfer of a resistance gene can also occur through the spread of bacterially encoded phages. This method of introduction of a gene, termed transduction, is when genetic material is transferred by a virus from one cell to another. In this case, AMR genes, such as mecA and other genes conferring resistance to β-lactam antibiotics, have been shown to be present in phage particles so that transfer of these genes can occur.25,26
THE MISUSE OF ANTIMICROBIALS
The efficacy of antimicrobials, particularly antibiotics, has led to their overuse and misuse. Whenever antibiotics are used, a selective pressure is applied, and the bacteria that escape that pressure can then proliferate or transfer the resistance gene. This can occur when antibiotics are taken during a virus infection. Antibiotics are meant to target bacteria and therefore have little to no effect when it comes to viral infections. Therefore, the virus pathogen will be unaffected, but the bacteria existing in the patient’s body are being put through an antibacterial bottleneck.27 The same scenario can be true when antibiotics are incorrectly used during an allergy or for other nonbacterial agents. Examples of this could include a fungal infection (like athlete’s foot) or infection with a protozoan (like malaria). So, not only will the consumption of antibiotics select for resistant bacterial populations, the treatment will do nothing for the patient suffering from a nonbacterial infection. This can then lead to the rise of AMR bacterial pathogens. Ultimately, whenever it is possible, antibiotics should be prescribed only for a bacterial infection. It is critical that physicians and others with the authority to prescribe antibiotics should do so based on confirmatory medical laboratory tests and antibiotic susceptibility panels.28
URBANIZATION AND INTERNATIONAL TRAVEL AS DRIVERS FOR AMR
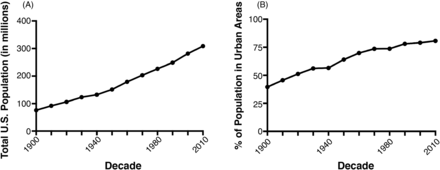
Accelerating the spread of AMR pathogens is a compaction of the human population into large metropolitan areas or urbanization. The rate of urbanization of the human population in the United States is quite staggering. At the time of WWII, when antimicrobials, such as penicillin, burst onto the scene, only about 50% of the US population lived in what is designated an urban population. This has jumped up to approximately 80% in the most recent decade and is showing no signs of slowing down (Figure 2A). Couple this urbanization with a robust population increase in the United States (Figure 2B), and you begin to see a picture of just how abrupt this shift has become. Not only has the United States seen a tremendous population growth, the proportion of that growth localized to urban areas has increased. This can be a breeding ground for pathogens.
The rise in the population in the United States and urbanization. (A) The United States has seen triple the population growth in the past 100 years. (B) The percentage of the US population living in urban areas has increased dramatically in the past 100 years. All data was obtained from the publicly accessible www.census.gov.
One of the best examples of a high-human-density pathogen is Mycobacterium tuberculosis (Mtb). Mtb is the causative agent for tuberculosis, in which Mtb colonizes the lung and is spread from human to human by coughing. Although it is one of the most prevalent bacterial human diseases in the world (thought to infect approximately one-quarter of the human population), Mtb is a surprisingly difficult pathogen to transmit.29⇓⇓-32 One of the factors that has facilitated its continuous spread in the human population is the urbanization of humans, particularly in the Eastern hemisphere.30,32,33 Although it is not at all surprising that those who encounter patients harboring Mtb are at a greater risk for contracting it, it is this continuous exposure that allows the pathogen to maintain an almost unparalleled level of success in human transmission. Also, given the disease burden (>1.5 billion currently infected), AMR of Mtb has become an increasingly burdensome health issue. Approximately one-third of all deaths attributed to Mtb infections are linked to AMR.30,34 A particularly worrisome development of Mtb is a class termed “extensively drug resistant,” in which the bacteria have developed AMR to a number of antibiotics.31,34⇓⇓⇓⇓-39 This typically arises through the misuse of prescribed antibiotics, in which the patient skips a treatment or does not finish their antibiotic regiment, allowing the flourishing of bacteria that were not immediately killed. This population has been previously exposed to the antibiotics, indicating that it will be less likely to respond to further use of that particular drug.
Along with the increase in urbanization, it is impossible to ignore the effect of accessible and rapid interstate, intercountry, and intercontinental travel on AMR. An excellent example of this is the spread of HIV. HIV likely emerged into the human population in the early 20th century from the ancestral simian immunodeficiency virus.40⇓⇓-43 Although this emergence of HIV took place in Africa, it quickly spread throughout Europe and into the Western Hemisphere by the 1970s. Much of this spread was likely facilitated by the rapid rise in trade and intercontinental travel. The virus then quickly established itself in the United States in populous-dense cities, such as San Francisco and New York City, among others.12,40,43 Since then, HIV has become one of the foremost human health crises, with current estimates of 1.8 million annual new infections and >1 million annual deaths from acquired immunodeficiency syndrome, even after the revolutionary highly active antiretroviral therapy.44 Complications with coinfecting agents, such as Mtb and its own AMR potential, have been an exceptionally troublesome issue, particularly in developing countries.30,31,34,35
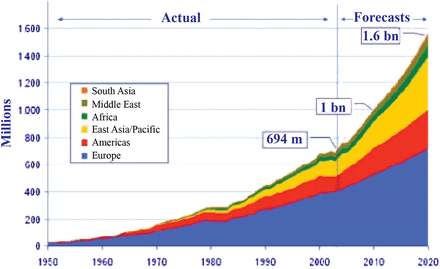
Of course, HIV is just one example of a veritable laundry list of pathogens that emerged into the population and rapidly escalated an epidemic with the help of human travel and urbanization. An increase in world travel and tourism was predicted in the early 2000s by the World Tourism Organization (Figure 3), and it has borne out with striking accuracy; this will continue to increase, as will the threat of intercontinental travel of AMR pathogens.
Increase in global travel and tourism, as predicted in the early 2000s by the World Tourism Organization.
ANIMAL CONTRIBUTIONS TO AMR
Not every pathogen to humans is exclusively transmitted from human to human. Many pathogens sustain enzootic cycles of transmission, such as rabies (rabies virus), Lyme disease (Borrelia bacteria), and the plague (Yersenia pestis). The ability for a pathogen to maintain an enzootic cycle creates a number of barriers for health professionals in terms of how to treat the disease. Should an outbreak occur in a community, patients can seek medical intervention. However, when the disease is cleared from the population, it still can maintain a cycle of transmission outside of humans and continuously spill over into the human population at a later time. Yersinia pestis is a prime example of this type of pathogen, as it has seen fewer cases of human incidence in recent decades; however, the bacteria still exist in fleas and animals. Because of this, the acquisition of AMR can be more difficult to track. In the case of Y. pestis, resistant strains have been identified.45,46
ENVIRONMENTAL CONTRIBUTIONS TO AMR
It may appear to the casual reader that AMR and global warming are distinct fields of science. However, global warming has significantly contributed to the spread of infectious diseases, particularly to the northern hemisphere.47,48 A good example of this is the increase in Vibrio-related infections in humans and marine life in the past few decades.49 Vibrio is a genus of gram-negative bacteria frequently found in unsterilized water. Cholera is caused by Vibrio cholera, which is acquired by the consumption of fecal-contaminated water and infects millions each year.50 Other Vibrio species, such as Vibrio vulnificus, can be acquired through the consumption of raw/undercooked oysters and shellfish.49,51,52 Unsurprisingly, the spread of Vibrio bacteria due to increases in ocean water temperature has coincided with increasing rates of AMR.49,51,53,54
Vibrio is just one example of a pathogen that seems to be riding the wave of climate change. Vector-borne diseases, such as West Nile virus, have been proposed to have shifted more north in response to global warming. This is due to the migration of the disease vector (in the case of West Nile virus, the Culex mosquito). Coupled with the urbanization in the Americas, vector-borne diseases can establish new niches in highly populated regions.55⇓⇓⇓-59 Through the introduction of novel pathogens into sustainable environments for their continuous transmission, the development of AMR is inevitable.
WHERE TO GO FROM HERE
Certainly, population mobility is a driver in globalization of public health threats and risks, specifically distribution of AMR organisms. Resistance to the majority of drug classes is a major present and future risk in both healthcare and community settings. Too often, our health policy paradigms in the past have focused on diseases of global public health impact, such as measles, influenza, tuberculosis, yellow fever, and cholera; however, new and emerging diseases like Zika virus and resistant organisms, such as the growing problem with multidrug-resistant gram-negative bacteria, challenge our traditional national and global strategies. Public health and clinical policy challenges associated with population mobility across global regions where pathogenic microbes, commercial and agricultural goods, and toxins (eg, geopolitical borders, patient care environments) are increasingly difficult and politically challenging. How do we measure the benefit versus risk of the travel in this context? Our existing travel systems, international law, and policies must be rethought and designed to slow down and prevent adverse health outcomes.60
According to a recent report by the World Bank Group, drug-resistant infections have the potential to cause a level of economic damage similar to and likely worse than that caused by the 2008 financial crisis. Key findings of the report based on World Bank Group projections of the world economy in 2017–2050 include impacts on the following concepts: global gross domestic product, global trade, global poverty, livestock output, and healthcare. Overall, it estimates a global economic impact of $100 trillion dollars and a human impact of 10 million deaths.61 A recent study by the University of North Carolina concluded that individuals who did not get vaccinated against the most common preventable diseases cost the US economy >$7 billion per year, with the flu contributing the largest portion of this.62 Given that this economic burden is placed on us by infections that can be prevented, one can imagine the cost of epidemics of new diseases, particularly those with AMR.
The rise and spread of AMR pathogens are not going to cease. As long as there are antimicrobial pressures, pathogens will continue to adapt. To that end, many resources are being paid to the development of novel antimicrobials. Moreover, vaccine development continues to be a priority for several of the infectious diseases we have outlined above and many others. However, given the long timeframe from development to Federal Drug Agency approval for vaccines,63 the need for new antimicrobials to combat existing infections is pressing. Therefore, targeting these AMR pathogens is truly like attempting to hit a moving target. Microbes do not read books or pay attention to public health policy, and they certainly do not follow the rules. Like the very AMR pathogens we are fighting, we must all become better at evolving and adapting our approaches in a more timely and urgent manner.
- Received May 21, 2018.
- Accepted May 22, 2018.
American Society for Clinical Laboratory Science