This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Society to Improve Diagnosis in Medicine
- Health Partners Medical Group and Clinics and Regions Hospital (retired)
- State University of New York Upstate Medical University (retired)
- Address for Correspondence: Paul L. Epner
, Society to Improve Diagnosis in Medicine, paul.epner{at}improvediagnosis.org
- CCCLW - Coordinating Council on the Clinical Laboratory Workforce
- TMTV - Taskforce to Measure Testing-Related Value
INTRODUCTION
A longtime parochial view of the laboratory is its lack of visibility and a concisely articulated and widely recognized understanding of its value to patients, providers, and government agencies.1 This jaundiced view of laboratory services is further affected by an almost singular focus on reducing costs and placing an emphasis on the clinical laboratory as a cost center. Yet, laboratory service expenditures account for less than 2.3% of national health costs in the United States, and only 2% of Medicare expenditures.2⇓-4 Furthermore, laboratory medicine provides value to society at large through accurate and timely biomarker measurements that aid in the diagnosis, monitoring, and support of wellness-centered patient care. Yet, the fiscal constraints imposed on the clinical laboratory threaten to further limit the value of the services it provides.
To address these concerns, a coalition of more than a dozen laboratory organizations spanning the breadth of the field of laboratory medicine came together to form the Coordinating Council on the Clinical Laboratory Workforce (CCCLW) with a threefold mission5:
• To increase the number of qualified clinical laboratory professionals.
• To enhance the image of clinical laboratory professionals.
• To increase healthcare and public awareness of our value in achieving positive patient outcomes.
A first step in promoting the value of laboratory medicine in patient care and outcomes entails the development of interventions that make a positive difference accompanied by measures that are sensitive to incremental benefit.6 Accepted measures of value that laboratory medicine contributes, or could contribute, through its professionals and related technologies would be important to health system decision-makers in allocating resources. Data collected from appropriately validated measures would drive insights that could guide development of a balanced approach to the twin needs of operational efficiency (ie, productivity and defect reduction) with clinical effectiveness (ie, improved patient and system outcomes).
To address the public awareness of the clinical laboratory’s contributions to health care, the CCCLW embarked on developing the necessary framework that would lead to the needed measures. The CCCLW commissioned the Taskforce to Measure Testing-Related Value (TMTV) and charged them to identify measures that correlate specifically to the value of laboratory services. The taskforce conducted a literature search, developed and disseminated a survey, and convened expert panels. This manuscript is focused on the survey—its development, dissemination, and results analysis.
METHODS AND MATERIALS
Survey Development and Dissemination
While laboratorians are increasingly being asked to justify their value, few submit their strategies to peer-reviewed publications. To identify novel approaches, a survey was developed to scan the landscape for evidence of initiatives, measures, and innovative thinking about laboratory value. The survey link was provided to the organizational members of CCCLW for dissemination to their membership. The format of the member notification varied by organization, from a dedicated email to a mention in a routine distribution of news. Individuals who clicked on the link were taken to SurveyMonkey, where they were able to complete the survey. Responses were collected from January 3 through January 31, 2016. Because the goal was to identify all relevant initiatives rather than develop generalizable knowledge, no attempt was made to eliminate multiple respondents from the same institution, nor to use a statistically representative sample.
The survey itself addressed 4 topics, along with the collection of demographic information. A copy of the complete survey is included in the supplemental information online (Appendix A).
Initially, questions about health system contexts that assessed leadership pressure to address value and separately to address cost were presented. Respondents were then asked to describe implemented initiatives that clearly demonstrated the value of laboratory medicine to patients and/or to the larger health system. The question explicitly stated that the initiative could have originated in the laboratory department or in any other department within the system. However, to be included, the value must have been acknowledged by non-laboratorians and the benefits had to be realized by patients or the health system and, if the latter, could not be limited to measures of internal laboratory quality or department-specific cost-effectiveness. The third topic sought to uncover measures used to evaluate the impact of this initiative as a demonstration of laboratory value. Finally, respondents were asked to identify “wishes” that, if available, would clearly demonstrate the value of laboratory medicine for both patient and health system outcomes. The final 3 topics were presented as open-ended questions to be completed in free-text fields.
The open-ended questions were analyzed by an initial full review of all survey responses, which led to the development of categories, subcategories, and a coding methodology that tied back to the developed taxonomy for each question. A code was only defined if at least 2 responses could be assigned to it. During a second reading, each response was assigned one of the available codes.
SURVEY RESULTS
A group of 469 individuals reflecting multiple interests in directly or indirectly providing laboratory services participated in the survey. Analysis of respondent demographics demonstrated participation from all 4 regions of the United States, as defined by the U.S. Census Bureau, as well as a broad range of laboratory disciplines (about half from the core laboratory), size of facilities (with similar proportions from large and small hospitals), and settings, including physician offices, clinics, hospitals (nearly 2/3), government facilities, reference laboratories, and independent laboratories. Detailed demographics are available in the supplemental information online (Figures S-1 through S-5).
Responses regarding initiatives taken that best describe the laboratory’s value.
Response to Change
Slightly more than 1/2 of the respondents agreed or strongly agreed that a shift from a structured fee schedule to capitated or value-based reimbursement was impacting system strategy and that it would cause a significant change in laboratory operations, as shown in Table 1. More than 2/3 of the respondents felt the need to prove the laboratory’s value to senior leadership, with a slightly higher number indicating the priority was cutting costs.
Completed Initiatives that Demonstrate the Value of Laboratory Medicine
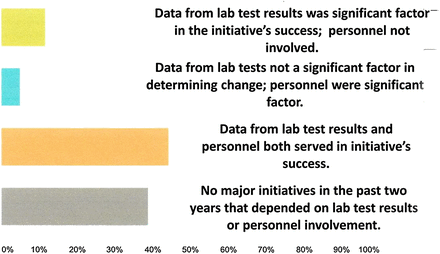
Respondents were asked to provide existing and/or completed quality improvement initiatives which linked the value of laboratory medicine to patient care and well-being. Reported initiatives from 133 coded responses fell into 6 categories, as noted in Table 2. Detailed responses within each category are available in the supplemental information online (Table S-1). The responses reflect current approaches laboratorians are taking to enhance the value of laboratory medicine. It is important to note that these results generally reflect quality improvement activities and demonstrate the transformation from a laboratory-centric to a collaborative patient-centric paradigm. And, in fact, if the traditional “Operational and Process Improvement” and “Quality Assurance Systems” sections are disregarded, 74% of the remaining initiatives point to a focus on collaboration between laboratorians and other health care professionals to jointly develop multidisciplinary improvement opportunities. The results of the collaborative efforts demonstrate value not only from laboratory technology, but also, importantly, from laboratory professionals (Figure 1).
Responses to change
Categories of current and completed quality improvement initiatives that demonstrate the value of laboratory medicine
Laboratory Measures on Patient Outcomes
Measuring the value of laboratory medicine has long been an elusive goal. When asked for the measures on which laboratorians rely, 184 codable responses fell within 6 categories, as summarized in Table 3. Specific responses within each category can be found in the supplemental information online (Table S-2). It was difficult to fully assess the nature of the measures solely from survey responses.
Examples of qualitative and quantitative measures that impact patient outcomes in determining the laboratory’s involvement and performance
Still, using a Donabedian framework,7 it appears that they are largely structural and process-oriented measures, sometimes limited to a narrow portion of the total testing process, eg, within laboratory turnaround times. This high concentration of structural and process measures limits the ability to prove causality on improved patient and system outcomes. The most outcome-oriented measures were associated with antimicrobial stewardship and blood transfusion management, which probably represents the low-hanging fruit of the value discussion.
Program Wishes to Demonstrate the Value of Laboratory Medicine
An established brainstorming technique (wishing) was used to elicit creative, potential approaches to novel programs/initiatives/interventions that could strongly demonstrate the value of laboratory medicine. The question was intended to help identify opportunities for improving outcomes and may suggest a path forward in achieving relevant value-based goals. The responses are divided into 7 categories, as detailed in Table 4. Specific ideas provided are listed in the supplemental information online (Table S-3).
Wish list concepts that could support the value of the laboratory
The following is a summary of the aggregated wish list responses:
• Clinical appropriateness and associated clinical decision support should be defined and function as a collaboration between laboratory personnel and clinical professionals and patients.
• Laboratory testing should be accurate and have proven clinical utility and effectiveness in patient management. Testing should also be accompanied with appropriate safety nets for prevention of errors in the preanalytical, analytical, and postanalytical phases of testing.
• Laboratory reports should be communicated to the ordering provider and patients as deemed necessary for effective and timely application in the care of the patient.
• Laboratory results should be easy to access, read, interpret, and document recommended follow-up. Coordination of care should be the outcome, with real-time access to laboratory professional clinical consultative support.
• Other safety nets as applied to monitoring and support systems, whether manual or electronic, should be collaboratively developed and implemented. This will allow clinicians, laboratorians, healthcare staff, and patients to easily make informed decisions about effective patient management.
• All the needed actions and safety support should be appropriately funded, with clear expectations for use and accountabilities. The balance measure of cost-effectiveness should be applied to all initiatives.
• Patients should be supported to allow easy engagement with the development of their care plans, including setting expectations for quality of life and the cause-and-effect of their actions.
DISCUSSION
More than 7 billion laboratory tests are performed annually in the United States.4 More than 4,000 different tests are available for clinical use, and approximately 500 of the more than 1,162 tests reimbursed by Medicare are performed on a regular basis.2 In addition to being used as indicators of wellness, diagnosis, and patient treatment management, the results from testing are used in public health surveillance, in therapeutic and diagnostic clinical trials, and in research.
Laboratory testing contributes to health management decisions from birth until the end of life. Approximately 4 million newborns are screened by tests for congenital and metabolic disorders annually.8 For the 5,000 infants with severe disorders, more specific testing, and, for some, life-long monitoring by laboratory tests will follow. Adults are accustomed to periodic screening tests such as cholesterol, fasting blood glucose, fecal occult blood, urinalysis, Papanicolaou/human papillomavirus testing, and prostate-specific antigen tests. Not only are test results incorporated into specific clinical guidelines, but a majority of adults expect and desire these as part of their routine physical examinations.9
Yet, despite these well-established uses for laboratory medicine, the clinical laboratory’s role is still generally seen as a cost center where expenses should be minimized and the understanding of value is limited to the need for accurate, timely, and low-cost results without regard to demonstrating improved patient outcomes.10 Furthermore, discussions of value are often limited to the diagnostic role of a specific analyte independent of the actual or potential contribution of the laboratory professional. The survey attempted to identify strategies for changing the discussion, yet the results spotlighted the limited efforts that were being made. In fact, the results point to just a few interventions where multiple responders identified the same focus areas.
Where results were identified, they were often linked to activities that were both multidisciplinary and collaborative, spotlighting the importance of laboratorians joining the broader care delivery team.
The importance of developing standardized processes involving protocols and best practices for diagnosis and treatment of disease states was also a common theme.
For example, initiatives related to infection prevention and therapy, in addition to providing overarching antibiotic stewardship, allow laboratory leaders to dynamically partner with other stakeholders to research and implement new technologies that can lead to faster and more accurate diagnosis and treatment. Accelerating identification of microbes and the application of local antimicrobial susceptibility testing to certain drugs (antibiograms) led to targeted and more effective antimicrobial therapies.11
Genetic testing in diagnosis, prognosis, and treatment was raised as an evolving area that will require collaborative teams to understand and apply this new knowledge for better patient care and outcomes. Personalized medicine should also help to engage the patients through recommended lifestyle changes.
Such initiatives as blood transfusion management and the antibiotic stewardship measures are evolving to be relevant laboratory medicine metrics. The transformational shift from producing simple biochemical results to distributing clinically meaningful and actionable insights is a critical component of a strategy that focuses on improving patient outcomes.
This research had several limitations. The respondents represent a convenience sample. Although dissemination occurred nationally, and the 469 responses exceeded expectations, no attempt was made to ensure the sample was representative nor generalizable to laboratorians nationally. Coding of responses was completed by one reviewer without a predefined protocol. The qualitative, open-ended nature of the responses limited the analysis that was possible. As such, the use of respondent’s exact wording is available in the supplemental material to assist readers in the interpretation of this information. Despite these limitations, there was sufficient commonality of responses with substantial diversity among the respondent’s demographics, that the broad conclusions made are deemed reasonable and informative.
CONCLUSIONS
Though medical laboratory testing is ubiquitous in practice, the ability to precisely quantify laboratory medicine’s actual contribution to patient outcomes, both short-term and long-term, remains challenging and relies mostly on anecdotal reports. Yet it is critically important that we engage in promoting the laboratory’s relevance and communicate the value that certainly exists. Increased investment in the laboratory and its personnel from healthcare leaders is crucial to ensure up-to-date equipment, the availability of sufficient qualified medical laboratory professionals, the opportunities to develop and implement new laboratory tests, and the pursuit of quality strategies and practices that maximize contributions from this critical field.
The value and benefits of quality laboratory services are ultimately received by the patient, yet there are other stakeholders dependent on the laboratory to maximize patient benefits. As reported elsewhere, there are 5 domains that best capture these stakeholders and their needs: (1) the needs of the patient’s care team in order to provide quality care to the patient, (2) the needs of the health system to ensure efficient and effective care and to prevent omissions of care or iatrogenic harm, (3) the needs of the patient in obtaining and understanding diagnostic information to facilitate their engagement, (4) the needs of the community-at-large in managing the public health burden, and (5) the needs of the laboratory professionals to learn from each other thereby speeding the spread and uptake of effective interventions.10
Efforts to increase public awareness of the value of laboratory medicine and its impact on all aspects of medicine are encouraging. The evolving complexity of laboratory medicine and its impact on health care is substantial. Failure to effectively articulate the value that is known, and the value yet to be described, poses risks for health care in that investment decisions will not be optimized for the opportunities that exist. It is important that clinical laboratory professionals adapt and build on work such as this so that the discussion of the contribution of laboratory services and information is understandable and useful.
- Received December 6, 2018.
- Accepted February 4, 2019.
American Society for Clinical Laboratory Science