This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Michael A. Nardi
, New York University of School of Medicine, michael.nardi{at}nyumc.org
LEARNING OBJECTIVES:
1. List and describe functional assays for hemophilia A and B.
2. Explain assay discrepancies for factor replacement therapy.
3. Detail the identification of inhibitory antibodies for factor replacement therapy.
ABSTRACT
This review describes and discusses the different types of factor assays used in the clinical laboratory for the diagnosis, as well as during therapeutic management, of hemophilia patients. After diagnosis, infusion of factor replacement concentrates is the standard of treatment for patients with hemophilia to prevent or treat bleeding episodes. Monitoring of the factor activity is necessary to assure proper treatment. Several assays are used to assess the factor activity at the time of diagnosis and during therapeutic management. The pros and cons of each assay are discussed in detail. With the introduction of many new replacement products, it is important the clinical laboratory understands the advantages and disadvantages of each assay with respect to each of the replacement products available.
- AHA - acquired HA
- APTT - activated partial thromboplastin time
- BDD - B-domain deletion
- CaCl2 - calcium chloride
- CSA - chromogenic substrate assay
- EHL - extended half-life
- FVIII - factor VIII
- FIX - factor IX
- FIXa - activated FIX
- FX - factor X
- FXa - activated factor X
- FXIa - activated factor XI
- HA - hemophilia A
- HB - hemophilia B
- N8-GP - glycopegylated Novo Eight
- OSA - one-stage assay
- pd - plasma-derived
- PL - phospholipid
- pNA - para-nitroanaline
- r - recombinant
- WHO - World Health Organization
- WHO IS - WHO International Standards
- one-stage assay
- two-stage assay
- chromogenic substrate assay
- hemophilia A
- hemophilia B
- replacement products
INTRODUCTION
Hemophilia A (HA) and B (HB) are congenital bleeding disorders associated with bleeding into joints and muscle tissues and are characterized by absent or reduced quantity or dysfunction of coagulation factor VIII (FVIII) and factor IX (FIX), respectively. The severity of the disease is closely correlated with the factor’s plasma activity level. Administration of replacement factor concentrate forms the standard treatment in patients with severe hemophilia (<1% factor activity), with nearly all hemophiliac boys in the United States, Canada, Australia, and Northern Europe receiving prophylactic treatment, resulting in reduced morbidity and mortality. The past decade has seen an explosion of new treatment options, including recombinant clotting factors engineered to extend their half-lives. Several of these extended half-life (EHL) FVIII and FIX concentrates have recently been approved, and others are in clinical trials. Although coagulation factor activity is measured for initial diagnosis, it is mostly measured in the clinical laboratory for clinical management at regular follow-ups, after infusion of factor concentrate, during preoperative and postoperative procedures and screening of inhibitor development. The one-stage assay (OSA) employing the activated partial thromboplastin time (APTT) is the most widely used assay in clinical laboratories. The two-stage chromogenic substrate assay (CSA) is an alternative assay, which has been available since the 1980s for FVIII and more recently available for FIX. The modifications applied to these molecules have introduced variations in their activity measurement in routine factor assays. Performance characteristics of the OSA and CSA for quantitation of FVIII and FIX at the time of diagnosis and after infusion of the plasma-derived (pd) factor concentrate (pdFVIII and pdFIX) as well as recombinant® factor concentrates without (rFVIII and rFIX) or with EHL (EHL-rFVIII and EHL-rFIX) have been reported, with many questions still outstanding. Thus, the clinical laboratory is now challenged to appropriately, accurately, and reproducibly measure factor activity levels for the infusion products given to patient. This article will focus on the measurement of FVIII and FIX activity in the clinical laboratory setting, with particular emphasis at diagnosis and in postinfusion plasma.
LABORATORY MEASUREMENT OF FVIII AND FIX
Functional Assays
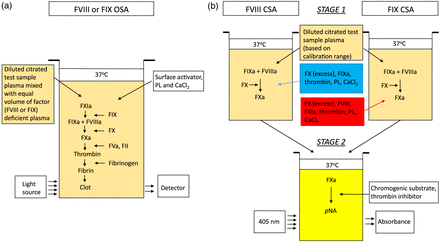
The two different functional assays used for quantification of FVIII and FIX are the OSA and the CSA.1 The OSA measures the ability of patient plasma to reduce the APTT clotting time of FVIII- or FIX-deficient plasma (obtained from congenitally deficient patients or immunodepleted normal pooled plasma) (Figure 1). Different dilutions of patient plasma are added to deficient plasma and preincubated with an APTT reagent containing phospholipid (PL) and a contact activator (eg, silica, ellagic acid, kaolin, polyphenols). After a set incubation time calcium chloride (CaCl2) is added, and the time to fibrin clot formation is measured according to the analyzer’s methodology and algorithm. The result is derived from a standard curve generated with a reference plasma of known FVIII and FIX concentrations. The large number of APTT reagents, deficient plasmas, reference plasmas, instruments, technical standardization procedures, and combinations thereof results in coefficients of variance of up to 40% and underestimation of factor activity by specific reagents.2⇓-4
Schematics of FVIII and FIX activity assays. (A) One-stage clotting assay. (B) Chromogenic substrate assay. CaCl2, calcium chloride; CSA, chromogenic substrate assay; FII, prothrombin (factor II); FIX, factor IX; FIXa, activated factor IX; FVIII, factor VIII; FVIIIa, activated factor VIII; FX, factor X; FXa, activated factor X; FXIa, activated factor XI; PL, phospholipid; pNA, para-nitroanaline.
The CSA for FVIII is a two-stage assay (Figure 1). In the first stage, a reagent containing purified activated FIX (FIXa), factor X (FX), and thrombin, as well as PL and CaCl2, are added in optimal concentrations to the patient’s plasma, resulting in the generation of activated FX (FXa). This step does not require additional coagulation factors because of the direct activation of FVIII by thrombin. In the second stage, the amount of generated FXa is measured by cleavage of an FXa-specific chromogenic substrate releasing para-nitroanaline (pNA), which causes a color change detected spectrophotometrically at 405 nm.1,5,6 Available commercial CSA kits for FVIII differ in source of purified proteins, activation process for FVIII, sample diluent, plasma dilution, CaCl2 concentration, and incubation times (Table 1). There are limited studies comparing CSA kits for sensitivity of FVIII measurement in different subgroups of HA.7,8
List of available chromogenic substrate assay kits
The CSA for FIX is also a two-stage assay (Figure 1). In the first stage, diluted patient plasma is mixed with a reagent containing purified FVIII and FX. The reaction is initiated with the addition of a mixture of activated factor XI (FXIa), thrombin, PL, and CaCl2. FXIa activates FIX to FIXa, and thrombin activates FVIII to activated FVIII (FVIIIa). FIXa and FVIIIa in the presence of PL and calcium activates FX to FXa. In the second stage, the amount of generated FXa is measured by its specific activity on a FXa chromogenic substrate that is cleaved, releasing pNA, which causes a color change detected spectrophotometrically at 405 nm.1,4⇓-6 Available commercial CSA kits for FIX are listed in Table 1.1,5,6
The two-stage clotting assay is a labor-intensive assay that is generally performed manually. It is presently only performed in very few specialized laboratories and will not be discussed.1,2
Assay Discrepancy in Plasma Samples
In a large proportion of untreated patients with moderate or mild forms of HA, a systemic discrepancy between the OSA and the CSA for the diagnosis of HA has been reported.5,9⇓⇓⇓⇓⇓⇓⇓⇓-18 Several reports have demonstrated discrepant results associated with specific causative FVIII gene mutations.1,16,17,19⇓⇓⇓⇓-24 The incidence of discrepant results has been reported to be 12%–50%.16⇓⇓⇓⇓-21 Discrepancy has not been found between OSA and CSA assays in patients with severe hemophilia.1 As an example, in a study of 163 HA hemophiliacs, 32 (20%) belonging to 16 families had discrepant FVIII levels, defined as either the OSA or CSA being at least 1.5 times higher than the other. Twenty-four patients (15%; 10 families) showed OSA results greater than CSA results, and 8 patients (5%; six families) presented CSA results greater than OSA results. Six of the patients in the latter group had CSA results within the normal range. In patients with discrepant results, other members of the family showed similar laboratory data. The bleeding tendency and the FVIII and/or DDAVP consumption correlated with OSA result. Mutation analysis showed a strong relationship between the phenotype and the mutation here and elsewhere, suggesting a genetic basis for the discrepancy between OSA and CSA results.19⇓-21 This study shows if only the OSA is used for screening of patients with mild HA, some patients will seem to have normal FVIII activity levels and not be diagnosed as having HA or will be assigned to a milder bleeding risk than is the case, increasing the risk of bleeding complications in a subsequent surgery without proper hemostatic treatment. Conversely, on rare occasions patients with normal FVIII activity in vivo will be diagnosed as having HA, risking cancellation or delay of necessary surgery or exposure to unnecessary factor concentrates or blood products.1
In mild HB, the CSA is higher than OSA, with the CSA results more closely correlating with bleeding phenotype.26 For HB, only a single report could be found investigating the discrepancy between OSA and CSA on HB patient plasma samples. In this report from a single center, 50 plasma samples from 36 patients were analyzed. Patients with severe HB showed no discrepancy in results. Among the 44 plasma samples from 32 patients in 18 families with mild or moderate HB, 15 samples from 8 patients (25%) showed a CSA at least two-fold greater than the OSA. Of these 15 samples, 14 were from 7 individuals from 5 families with 2 different nucleotide substitution at the same amino acid (6 with p.Arg191His and 1 with p.Arg191Cys) in the N-terminal cleaving site of the activation peptide. The mutations were not observed in patients with nondiscrepant results. For patients with the Arg191His mutation, the OSA method result was 2% for all and a range of 5%–8% (p = 0.001) for the CSA method. A detailed review of the medical records for these patients revealed 5 patients on on-demand treatment and one younger patient on prophylaxis with no history of spontaneous bleeding episodes. Only one of these patients has experienced a spontaneous bleed (hematuria) requiring replacement therapy. The single patient with the Arg191Cys mutation had FIX levels of 1% and 5% by OSA and CSA, respectively. This patient was reported as being currently on on-demand treatment but has been on short-term prophylaxis due to episodes of epistaxis. The authors interpreted the bleeding frequency for these patients as low and indicative of a mild bleeding phenotype and state their findings imply assay discrepancy occurs frequently and both OSA and CSA assays are needed for correct diagnosis and classification of HB.25
Replacement Products for Clinical Treatment
In addition to the diagnosis of hemophilia, factor activity measurement is needed for monitoring of factor levels in prophylactic therapy and during treatment of acute bleeds, determination of the pharmacokinetics, and successful perioperative management of factor replacement therapies. Prophylactic therapy is presently the standard of care in the developed world for the severe form of hemophilia and consists of frequent infusion of a factor replacement product, most commonly plasma-derived (pd) or recombinant (r) full-length factor concentrates. It has proven to be efficient in controlling bleeding episodes of patients with hemophilia; however, it requires frequent infusions (three to four times per week for FVIII and two to three times per week for FIX) to maintain factor values above the threshold of efficacy (generally >1%). It represents a serious burden that significantly impacts the patient’s quality of life.28 The last decade has seen the development of numerous modified products (EHL factor protein replacements) as well as alternative treatment modalities (alternative factor bypassing therapeutic products and gene therapy) for the treatment of hemophilia. The EHL factor protein replacements products are intended to allow a reduction in the frequency of infusion and improve the standard of care for people with hemophilia. Some of these products have received U.S. Food and Drug Administration approval (Table 2), whereas others are in clinical trials.26 Due to substantial interpatient variability in response to a replacement product, infusion adjustment of frequency and level of dosing is often required, resulting in the need for repeated assessments of factor levels in the clinical laboratory.
List of FDA-approved EHL products
Assay Discrepancy for FVIII Assays in FVIII Replacement Products
The OSA and CSA both accurately assess FVIII activity for pdFVIII. Assay discrepancies between OSA and CSA have been reported for full-length rFVIII products, with the CSA values 8%–20% higher than the OSA values,27⇓⇓⇓⇓-32 with the exception of B-domain deletion (BDD) rFVIII. This discrepancy is not considered clinically significant (ie, within the accepted variation of ≤20%).6 Reports on BDD-rFVIII, however, have shown discrepancies of 20%–50%, with the OSA lower than the CSA.28⇓⇓⇓-32 EHL recombinant factor concentrates have been shown to pose significant challenges to the laboratory monitoring of posttransfusion samples.33 These factor replacement products contain various molecular modifications (BDD/truncation, fusion with Fc region of IgG or with albumin, or linkage to polyethylene glycol) intended to extend the half-life. Many of the truncated products show wide variation in measured activity levels between different OSAs as well as between the OSA and CSA.9,34 However, not all such products have this issue. For example, glycopegylated Novo Eight (N8-GP), a B-domain truncated rVIII, appears to have acceptable recovery across various OSAs, with the exception of one reagent.35 Similarly, N8-GP levels are consistently measured across six distinct CSAs.36 A comprehensive review of assay performance among each EHL factor concentrate is beyond the scope of this paper and can be found elsewhere3,34,37 In general, OSA and CSA can vary their estimation of factor activity by 20%–50% for most products that could be clinically relevant, particularly in individuals undergoing treatment for a major bleed or surgery.
Assay Discrepancy for FIX Assays in FIX Replacement Products
Similarly to FVIII, the OSA and CSA both accurately assess FIX activity for pdFIX.37⇓-39 Differences between OSA and CSA have been reported with two full-length rFIX, with CSA results consistently about 70% of OSA results in two rFIX concentrates and in postinfusion samples with one of these products.39 This observation has been confirmed in another report on the evaluation of three CSA FIX kits.40 A recent study using samples containing glycopegylated rFIX showed FIX activity was underestimated by 30%–70% and overestimated by 500%–2800% depending on the reagents used in the OSA.41 Studies on FIX fused with Fc indicated accurate measurement of postinfusion plasma rFIXFc was APTT reagent activator-dependency in the OSA with ellagic acid resulting in overestimation, and silica (30%) and kaolin (50%) resulted in underestimation.42,43 Another study reported comparable results could be achieved between the CSA and the OSA if selected APTT reagents.44
It must be noted the vast majority, if not all, of data that have been published on the performance of different OSA reagents and the CSA kits on recombinant and EHL recombinant factor replacement products were collected using factor concentrates diluted to different concentrations in appropriate factor-deficient plasma. This data will need to be confirmed in clinical plasma samples collected postinfusion in patients with hemophilia.
Causes for Variability in the Factor Assays
Review of proficiency testing programs and field surveys demonstrates OSA is currently used in the majority of clinical laboratories for the clinical diagnosis of hemophilia and for monitoring factor replacement therapies.45⇓-47 The OSAs for FVIII and FIX activity use a wide variety of APTT reagents differing in PL source, type, concentration, and activator type. The activator type seems to exert a major influence on activity discrepancy within the OSA. Factor-deficient plasma may be from congenital-deficient hemophilia patients or, more frequently, factor-specific immunodepleted plasma. Other variables that have been shown to influence the accurate measurement of factor activity are the analyzer and its methodology, the assay protocol, and the calibration material.48,49
The potency labelling for FVIII and FIX concentrate products, including plasma-derived, recombinant, and EHL-modified products, is performed by the manufacturers, generally using either the OSA or the CSA. The ISTH/SSC has published recommendations for manufacturer’s potency labelling.50 These recommendations discuss establishment of valid estimates using OSA and CSA, OSA evaluation performed using different APTT reagents, and potency assessment performed relative to the World Health Organization (WHO) International Standards (WHO IS). The recommendations also state potency assessment should be conducted using a reference plasma, which would not necessarily be used for potency assignment but could be useful when considering the use of a plasma reference to monitor recovery of new products in the clinical laboratory as well as a plasma reference. These recommendations all directly pertain to improving the accuracy of testing in the clinical laboratory. Potency assessment for many of the FVIII replacement products uses CSA. Indeed, the FVIII and FIX subcommittee of the ISTH/SSC and the European Pharmacopoeia, which sets mandatory standards for medicinal products in Europe, defines the FVIII CSA for potency labeling of FVIII medicinal products; however, the OSA is generally preferred in clinical laboratories in Europe and throughout the world. Presently, there is no recommendation for FIX, presumably because the FIX CSA is only recently available. Some of the available FVIII replacement products have shown no discrepancy in results between OSA and CSA, whereas some products do show discrepancy. Almost all of the EHL-FVIIIs have shown discrepancy, which seems to be reagent dependent. The clinical laboratory must be well aware of this information. It should refer to the package insert and the published literature for guidance as to what methodology should be used for monitoring as well as the performance of its assays with a particular factor replacement product, especially the EHL products.
The optimal approach for measurement of factor activity involves the testing against a product reference composed of the same material as that which is infused and the same methodology, that is, a like-versus-like approach for potency assessment. Although patient plasma samples at the time of diagnosis and post–pd-factor replacement products are assayed versus a like-versus-like reference material traceable to the WHO IS for FVIII or FIX, this concept would be very difficult to implement for the recombinant and EHL factor replacement products. Reference and control material for the recombinant and EHL replacement products are not available. To allow appropriate interpretation of laboratory results, it will be necessary for the manufacturer to provide guidance to clinicians whose laboratories use OSA to measure FVIII and FIX activity of products clinically validated and labeled by use of the CSA.6 Also, the laboratory must be knowledgeable of the available replacement products as well as the ability of its laboratory assay to accurately measure those products and to communicate such information to the clinician.
INHIBITORS
The development of inhibitory alloantibodies following exposure to factor replacement therapy is a major complication of hemophilia. These antibodies are mainly IgG and bind to functional domains on FVIII or FIX, inhibiting its anticoagulant activity.51,52 Although inhibitors to FIX are uncommon, occurring in approximately 3% of HB patients, anti-human FVIII antibodies develop in approximately 30% of patients with severe HA following exposure to factor replacement. The presence of an inhibitor results in poor response to standard doses of clotting factor replacement.53 As a consequence, compared with patients without inhibitors, those with inhibitors have more frequent, poorly controlled hemarthroses resulting in more severe joint disease and are at greater risk for life- or limb-threatening hemorrhage and have reduced health-related quality of life.54⇓-56
De novo inhibitors to FVIII can also manifest in non-HA patients in a rare condition called acquired HA (AHA). The majority of AHA cases are idiopathic, whereas the remaining are associated with autoimmune disease, malignancy, pregnancy, and postpartum state.57,58 The bleeding phenotype of AHA differs from congenital HA, with soft tissue bleeding being more common than hemarthroses.
Inhibitory antibodies to FVIII can be identified in the laboratory using OSA, most commonly the Bethesda clot-based assay, or enzyme-linked immunosorbent assays.59 The classic Bethesda assay involves normal pooled plasma as the source of FVIII being incubated in undiluted patient plasma for 2 hours at 37oC and then assayed for residual FVIII. One inhibitor unit (Bethesda Unit, BU) is defined as the amount of patient plasma that destroys 50% of the FVIII in the mixture, corrected for the deterioration of FVIII in a control consisting of normal plasma incubated with buffer. Positive results may require further dilutions of the patient plasma to be assayed to obtain an accurate result.59⇓-61 The Nijmegen modification of this assay has been shown to increase the specificity of low-titer FVIII inhibitors. This modification involves buffering the normal pooled plasma used in patient and control mixtures to pH 7.4 with imidazole buffer and using FVIII-deficient plasma in the control mixtures for preparing patient dilutions. Both adjustments maintain the pH of the reaction mixture for the 2-hour incubation by stabilizing the FVIII in the pooled normal plasma.62 Additional modifications have been made and evaluated, including heating the patient plasma 56–58oC for 60–90 minutes to inactivate any circulating FVIII activity, the appropriated use of FVIII-deficient plasma, the use of 4M imidazole solution instead of solid imidazole for buffering, and use of 4% solution of bovine serum albumin in place of FVIII-deficient plasma. These as well as the use of CSA to measure the residual FVIII hopefully will continue to improve the laboratory’s ability to accurately and reproducibly measure FVIII activity.63⇓-65
Patients with hemophilia who develop inhibitors (neutralizing anti-drug antibodies) to their factor treatments are particularly difficult to manage. Successful immune tolerance induction is the best option and enables use of factor replacement therapy for treating acute or traumatic bleeds.53
Test Costs
A recent article by S. Kitchen et al utilized a computer-based cost analysis model to identify and characterize key cost parameters associated with OSA and CSA for both FVIII and FIX assays.64 The authors conclude that the key factors that contribute to costs are factor-deficient plasma and kit reagents for OSA and CSA, respectively. The stability of CSA kit reagents, especially the FIX kit, also limits the cost efficiency compared with OSA. The authors estimate the cost of CSA might be reduced 50%–75% using batch testing, aliquoting, and freezing of kit reagents; however, these changes would require validation. A recent publication by Bowyer et al reported that for using aliquoting and freezing and analyzing adequate number of controls in each run, the estimated cost for CSA was 21% more than OSA if 20 samples are analyzed in each batch and, interestingly, 8% cheaper if only one sample is analyzed.66 Other findings important to mention are the perception that CSAs are technically complex, the lack of validated protocols by the reagents’ manufacturers, and the cost of training.
CONCLUSION
HA and HB are congenital bleeding disorders, the diagnosis of which hinges on accurate assessment of FVIII and FIX, respectively. The current standard of care is prophylactic infusion of factor replacement products for the severe form (<1% activity) and infusion on demand for the moderate (1%–5% activity) and mild (5%–40% activity) forms of both HA and HB. Historically, the infusion was achieved first using human donor plasma factor concentrates and, later, recombinant forms of factor concentrates. Recently, replacement products have been introduced that contain modifications to the recombinant factors intended to extend their plasma half-life for the purpose of reducing the frequency of infusion. The OSA assay is the most commonly used test for assessment of FVIII and FIX activity in the clinical laboratory. The CSA is less frequently employed. For the diagnosis of hemophilia, reports have shown the OSA and CSA perform very well in detecting severe forms of HA and HB; however, discrepancy is found in the moderate and mild forms, especially the latter, of both HA and HB. Thus, a single method to measure FVIII and FIX may not be sufficient to detect all cases of this disorder. For the assessment of postinfusion factor activity in plasma samples with plasma-derived or recombinant FVIII and FIX replacement products, the OSA and CSA perform, with some exceptions, reasonably well. For example, with the introduction of BDD-FVIII, some assays became inaccurate using a human FVIII reference plasma. The “solution” to this problem was the use of a different reference material, requiring the laboratory to perform different FVIII assays for different purposes. With the new modified EHL-recombinant factor replacement products, discrepancies in results with different OSA reagents as well as between OSA and CSA have been reported. These reports indicate that, dependent on the replacement product monitored and the methodology used, substantial overestimating or underestimating of factor activity can occur, resulting in the potential for inappropriate patient management. Thus, the challenge for the laboratory to provide a diagnostic strategy that is sensitive, practical, and within budget allowances. To do so will require the clinical laboratory performing factor assays to review the FVIII and FIX assays presently provided in light of the hemophilia factor replacement products used in the medical service to which its service is provided. Good communication between the laboratories and the physician about the product the patient has received is of utmost importance. Looking to the future, any clinical laboratory that measures factor activity in hemophilia patients should gain the knowledge about the different factor replacement products, should participate in any dialogue with clinicians and whichever hospital department purchases and dispenses these products, and should determine whether their current method for determining factor activity provides an accurate measurement of that particular replacement therapy. For those laboratories that do not have the optimal measuring technique, the treating clinician must be informed about the potential biases and an alternative means of measuring accurate concentration provided.
- Received October 18, 2018.
- Accepted March 31, 2019.
American Society for Clinical Laboratory Science