This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Rush University
- Rush University Medical Center
- Rush University Medical Center
- Rush University Medical Center
- Address for Correspondence: Nadine Lerret
, Rush University Medical Center, nadine_lerret{at}rush.edu
ABSTRACT
Obesity is now strongly associated with chronic low-grade inflammation that, without intervention, contributes to the development of prediabetes and eventually type 2 diabetes. Although the exact mechanism that inflammation plays in the pathogenesis from obesity to type 2 diabetes is unclear, activated immune cells and proinflammatory cytokines have been found in the adipose tissue of people with type 2 diabetes, implicating their role in the disease process. The CD27-CD70 pathway provides a crucial inflammatory costimulatory signal, with CD70 being expressed on activated antigen-presenting cells and CD27 expressed on lymphocytes. Although the CD27-CD70 axis is being explored in other models of chronic inflammation, such as rheumatoid arthritis and colitis, the role played in type 2 diabetes remains unknown. This article reports the downregulation of CD27 on CD4 T cells when cocultured with dendritic cells primed in increasing concentrations of glucose, indicating an effector phenotype of these T cells. Importantly, it is also highlighted that CD70 is concurrently upregulated on dendritic cells primed in high concentrations of glucose, which results in increased production of interferon-γ and tumor necrosis factor α by the CD70 expressing dendritic cells when compared with dendritic cells primed in a lower concentration of glucose. These results reveal a novel role for CD27-CD70 interactions in the pathogenesis of type 2 diabetes and provide support for future investigations into this pathway. Additionally, CD27 could be analyzed to further stratify patients with prediabetes and guide diagnosticians towards the most efficient therapy.
- ADA - American Diabetes Association
- APC - antigen-presenting cell
- DC - direct current
- GM-CSF - granulocyte-macrophage colony-stimulating factor
- IFN - interferon
- IL - interleukin
- MACS - magnetic-activated cell sorting
- NK - natural killer
- PBMC - peripheral blood mononuclear cell
- PBS - phosphate-buffered saline
- TNF-α - tumor necrosis factor α
- TNFR - tumor necrosis factor receptor
- type 2 diabetes mellitus
- CD4-positive T lymphocytes
- inflammation
- hyperglycemia
- prediabetic state
- dendritic cell
INTRODUCTION
The most recent report from the Center of Disease Control1 unveils that a staggering 100 million Americans are now living with diabetes or prediabetes. Prediabetes is a disease that when addressed promptly and efficiently can be reversed. However, only 11.6% of those with prediabetes are aware of their prediabetic status and condition.1 The American Diabetes Association (ADA) defines laboratory values for prediabetes as hemoglobin A1c values between 5.7% and 6.4%, fasting plasma glucose values between 100 and 125 mg/dL, and/or an oral glucose tolerance test of 140–199 mg/dL.2 Furthermore, a study by the ADA found that approximately 10% of patients with prediabetes convert to full diabetes annually.3 Laboratory testing plays an important role in distinguishing between prediabetes and diabetes, which in turn helps in disease management and even possible reversal. With the previously mentioned studies bringing more attention and awareness to prediabetes and diabetes, efficient screening and patient stratifying protocols need to be standardized to prevent diabetes-associated complications and comorbidities.
Although the pathogenesis of type 2 diabetes is not fully understood, strong evidence now links chronic inflammation and activated components of the immune system, such as macrophages, dendritic cells, T cells, and cytokines, to the pathogenesis of prediabetes and type 2 diabetes. The laboratory has found that human CD4 T cells cocultured with dendritic cells primed in increasing concentrations of glucose show a dose-dependent effector phenotype with the highest concentrations of glucose inducing the highest level of CD4 T-cell activation.4 For activation, naïve T cells require interaction with antigen-presenting cells (APCs) along with T-cell receptors: major histocompatibility complex engagement and costimulatory signaling. CD27-CD70 is a well-studied costimulatory receptor ligand pair in the tumor necrosis factor receptor (TNFR) superfamily.5 CD27 is constitutively expressed on naïve CD4 T cells but is downregulated when T cells differentiate into effector cells.6 With expression largely limited to activated APCs, CD70 is tightly regulated and is the sole ligand for CD27.7 Infection and immunization models have identified a crucial role played by the CD27-CD70 pair in T-cell priming.8⇓⇓-11 However, this has not been investigated in diabetes and chronic hyperglycemia. This research, therefore, investigated CD27 and CD70 on human CD4 T cells and dendritic cells derived from peripheral blood mononuclear cells (PBMCs) in this hyperglycemia model. The research demonstrates that when cocultured in hyperglycemic conditions, CD4 T cells downregulate their expression of CD27, whereas dendritic cells simultaneously upregulate their CD70 expression. In addition, the inflammatory status of the dendritic cells was confirmed through their increased cytokine production, with both interferon (IFN)-γ and tumor necrosis factor α (TNF-α) found to be increased. These results indicate that in conditions with elevated blood glucose, such as diabetes, the CD27-CD70 axis is activated, and both CD4 T cells and dendritic cells exhibit a proinflammatory phenotype and function. This study provides support for future investigations into analyzing the expression of CD70 and CD27 on the surface of dendritic cells and CD4 T cells from the peripheral blood of patients with prediabetes or diabetes. The study also provides a mechanism to stratify the patients most at risk for inflammatory-related comorbidities or the rapid progression from prediabetes to diabetes.
MATERIALS AND METHODS
Cell Separation and Isolation of PBMCs
Isolation of human PBMCs was carried out using Histopaque (MP Biomedicals) density-gradient centrifugation. Human whole blood used for PBMC isolation was provided as a unit of deidentified whole blood from LifeSource Inc (Chicago, IL). The blood was diluted 1:2 with phosphate-buffered saline (PBS; MP Biomedicals) and layered over Histopaque (MP Biomedicals). This was then centrifuged at room temperature (20°C –25°C) for 30 minutes at 2000 rpm. The interface (buffy coat) was recovered and washed 3 times with PBS (MP Biomedicals). The resulting PBMCs were subject to positive selection of CD14+ and CD3+ cells via magnetic-activated cell sorting (MACS) technology. This study was exempt from institutional review board approval because all blood used was completely deidentified, and donors did give their informed consent to donate peripheral blood.
Immature Dentritic Cell and T-Cell Isolation From PBMCs
CD14 is a pan-monocyte marker that identifies PBMCs that can differentiate into APCs, such as macrophages and dendritic cells. Therefore, monocytes were purified from the isolated PBMCs by positive selection using anti-CD14–conjugated magnetic microbeads (Miltenyi Biotec). The PBMCs were incubated with the anti-CD14–conjugated microbeads for 20 minutes at 4°C and washed with MACS buffer (Miltenyi Biotec) before being applied to a MACS column (Miltenyi Biotec). The column was then washed 3 times with MACS buffer to wash away any non-CD14+ cells before the column was eluted for CD14+ monocytes (purity >85%). Concurrently, T cells were purified from the isolated PBMCs by positive selection using anti-CD3–conjugated magnetic microbeads (Miltenyi Biotec). A separate fraction of PBMCs were incubated with the anti-CD3–conjugated microbeads for 20 minutes at 4°C, washed with MACS buffer (Miltenyi Biotec), and then applied to a MACS column (Miltenyi Biotec). The column was then washed 3 times with MACS buffer to wash away any non-CD3+ cells before the column was eluted for CD3+ T cells (purity 87.6%). Both purity measurements were determined through flow cytometry.
Generation of Monocyte-Derived Direct Currents
Isolated immature CD14+ monocytes (1×105/mL) were cultured for 6 days in 6-well tissue culture plates containing glucose-free HyClone Laboratories Inc Roswell Park Memorial Institute media supplemented with 10% fetal bovine serum (Gibco), 1mM sodium pyruvate (Gibco), 0.1mM nonessential amino acids (Gibco), and 1% penicillin/streptomycin (Gibco). Conventionally, peripheral blood monocytes are differentiated into myeloid dendritic cells by adding granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 to the culture media.12 Accordingly, in this culture system, GM-CSF (100 ng/mL) and IL-4 (50 ng/mL) were added to the culture media of CD14+ monocytes to differentiate them into immature dendritic cells. Cells were incubated at 37°C with 5% carbon dioxide with media changed every 2 days. On day 6 of culture, the resulting immature direct currents (DCs) were subject to varying concentrations of glucose (Thermo Fisher Scientific) and incubated for an additional 24 hours to allow sufficient time for glucose stimulation. The conditions included complete absence of glucose or the addition of 5.5 mmol/L (physiologic), 15 mmol/L (prediabetic), or 30 mmol/L (hyperglycemic) glucose concentrations.
Dendritic Cell: T-Cell Coculture
Naïve autologous CD3 T cells (1×106/mL) were added to the cultured DCs after the DCs had been incubated with or without glucose for 24 hours. The culture medium was supplemented with IL-2 (80 U/mL; Thermo Fisher Scientific). It is possible that the dendritic cells used all of the glucose before the T cells were exposed; therefore, T cells cocultured with dendritic cells in the absence of glucose and provided a valuable internal control. For the positive control, T cells were stimulated with CD3/CD28 beads (Tonbo Biosciences) to mimic in vivo antigen presentation through T-cell coreceptor and costimulatory molecule engagement. The DCs and T cells were cocultured for 7 days before harvesting for flow cytometry assays.
Flow Cytometry Assays
Lymphocytes were stained with fluorochrome conjugated monoclonal antibodies directed against human CD3 (SK7), CD4 (SK3), and CD27 (O323), all from Tonbo Biosciences, and IFN-γ (B27), TNF-α (MAb11), and CD70 (Ki-24) from BD Biosciences. Monocyte-derived dendritic cells were stained with CD11b (ICRF44), CD14 (61D3), and CD11c (3.9) from Tonbo Biosciences and CD70 (Ki-24), IFN-γ (B27) and TNF-α (MAb11) from BD Biosciences. For intracellular staining, lymphocytes and dendritic cells were cultured for 5 hours with 1 mg/ml GolgiPlug (BD Biosciences) and then stained for surface markers (CD3 and CD4 for T cells and CD14, CD11b, and CD11c for dendritic cells). Cells were then fixed and permeabilized using Cytofix/Cytoperm Fixation and Permeabilization Solution (BD Bioscience) for 20 minutes at 4°C and were subsequently stained with anti–IFN-γ, anti–TNF-α, or the corresponding isotype controls (BD Biosciences). Finally, cells were washed twice and analyzed by flow cytometry. The combined use of cell surface markers with intracellular staining allows for the identification of cytokines being produced on a single-cell basis. Flow cytometry was performed on a BD LSRII Flow Cytometer (BD Biosciences), and data were analyzed with FlowJo LLC software.
STATISTICAL ANALYSIS
Data were expressed as the mean plus or minus SEM of independent experiments. Statistical significance was determined by a Student’s 2-tailed paired t-test assuming equal variances or analysis of variance. P values less than 0.05 were considered statistically significant.
RESULTS
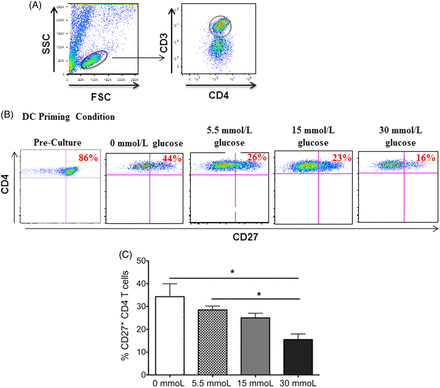
CD4 T cells have an activated effector phenotype after coculture with glucose-stimulated dendritic cells. The functional state of CD4 T cells can be divided into distinct subsets, naïve, effector, or memory T cells, based on their phenotype—that is, the expression (or lack of expression) of diverse cell surface receptors.13 A surface receptor that is frequently used as a marker of effector state or activated CD4 T cells is the TNFR CD27.14 Previous studies have shown a stepwise downregulation of CD27 on the surface of naïve CD4 T cells differentiating into effector and further into memory CD4 T cells.15,16 Therefore, the study evaluated the CD27 expression on CD4 T cells from coculture with glucose-primed dendritic cells. Because CD27 is highly expressed on naïve CD4 T cells, the analysis of CD4 T cells prior to being added to culture with dendritic cells was included as a comparison. In accordance with this finding, it was observed that the naïve CD4 T cells expressed high levels of CD27 (86%) prior to being added to the coculture (Figure 1A and 1B). Interestingly, flow cytometric analysis revealed a dose-dependent gradual decrease in CD27 expression on CD4 T cells in coculture with dendritic cells primed in increasing concentrations of glucose with the 30 mmol/L (hyperglycemic) priming condition, which showed the smallest percentage of CD27+ CD4 T cells (16%, Figure 1B and 1C). Although the dendritic cells that were primed in the absence of glucose also induced the downregulation of CD27 on subsequent CD4 T cells encountered, the data show a statistically significant decrease in CD27 expression between CD4 T cells cocultured with dendritic cells primed with 0 mmol/L or 5.5 mmol/L (physiological) of glucose and CD4 T cells cocultured with dendritic cells primed with 30 mmol/L of glucose (P = 0.033 and 0.027, respectively).
CD27 expression on CD3+CD4+ T cells in coculture with dendritic cells primed in increasing concentrations of glucose. Peripheral blood–derived CD3+CD4 T cells were cocultured with autologous dendritic cells primed in varying concentrations of glucose. After 7 days in culture, the expression of CD27 on the surface of the T cells was analyzed via flow cytometry. In this figure, (A) gating scheme of CD3+CD4+ T cells gated from total lymphocytes in cell culture, (B) representative dot plots of this CD27 expression, and (C) bar graphs are shown. Data represent 2 independent experiments with 3–6 wells per culture condition. *P ≦ 0.05.
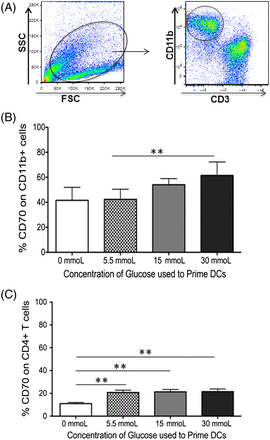
CD70 is upregulated on dendritic cells primed in hyperglycemic conditions. CD70 is also a member of the TNFR family and is currently the only known ligand for CD27, with CD27-CD70 acting as a costimulatory receptor ligand pair.17 Whereas CD27 is expressed on naïve and memory T cells as well as some subsets of B cells and natural-killer (NK) cells, CD70 is primarily expressed on APCs.10,18 Therefore, the expression of CD70 on the CD11b+ dendritic cells in the culture system was analyzed, primed in increasing concentrations of glucose. As shown in Figure 2A and 2B, there is an increase in CD70 expression as the concentration of glucose used to prime dendritic cells increases (P = 0.003). Although CD70 is primarily expressed on activated APCs, it can be expressed on activated T cells as well. Therefore, the T cells in coculture with the dendritic cells were analyzed for their expression of CD70. This analysis revealed similar expression irrespective of the concentration of glucose used in cultures (5.5–30 mmol/L, Figure 2C). However, T cells cocultured with dendritic cells in the absence of glucose reported a significant decrease in their CD70 expression (P = 0.002, 0.001, and 0.002 when compared with 5.5 mmoL, 15 mmoL, and 30 mmoL of glucose, respectively). Collectively, these data implicate the CD27-CD70 costimulatory pair to play a role in the activation of naïve CD4 T cells in hyperglycemic conditions.
PBMC-derived dendritic cells increase their CD70 expression when primed in increasing concentrations of glucose. Peripheral blood–derived human CD11b+ cells were primed in varying concentrations of glucose. Autologous CD3+ T cells were added at a 1:10 DC:T-cell ratio 24 hours after the addition of glucose. After 7 days in coculture, expression of the costimulatory molecule CD70 on CD11b+ dendritic cells or CD3+CD4 T cells was determined using flow cytometry. This figure includes (A) gating scheme of CD11b+ DCs gated from total cells in cell culture, (B) bar graphs showing the expression of CD70 on CD11b+ DCs, and (C) bar graphs showing the expression of CD70 on CD3+CD4+ T cells. Data represent 4 independent experiments with 3–6 wells per culture condition. *P ≦ 0.05; **P ≦ 0.01.
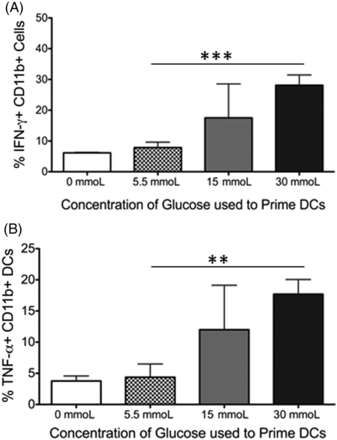
Dendritic cells produce IFN-γ and TNF-α when primed in hyperglycemic conditions. Although surface markers are one indication of the activation status of an immune cell, cytokines produced also give insight into the function of that cell. Therefore, it was examined whether the cells in the cocultures produced the inflammatory cytokines IFN-γ or TNF-α. In the coculture system, T cells produced little of either cytokine (data not shown). However, the dendritic cells displayed a dose-specific increase in their cytokine production as the respective priming glucose concentration increased (Figure 3A and 3B). This was true for both IFN-γ (Figure 3A, P = 0.001) and TNF-α (Figure 3B, P = 0.002) when dendritic cells primed in physiological glucose (5.5 mmoL) and hyperglycemic conditions (30 mmoL) were compared. Together, these results confirm that dendritic cells primed in a hyperglycemic environment display an activated phenotype and function, which results in subsequent activation of CD4 T cells, skewing them towards an effector/inflammatory phenotype.
PBMC-derived dendritic cells but not T cells secrete proinflammatory cytokines after 7 days of coculture in hyperglycemic conditions. Peripheral blood–derived human CD11b+ cells were primed in varying concentrations of glucose. After 7 days in coculture with autologous CD3+ T cells, the production of the inflammatory cytokines was analyzed from both cell types via intracellular staining. The figure shows (A) bar graphs showing the production of IFN-γ and (B) TNF-α from CD11b+ DCs. Data represent 2 independent experiments with 3–6 wells per culture condition. Unstained and isotype controls were used as negative gates for cytokine expression. **P ≦ 0.01; ***P ≦ 0.0001.
DISCUSSION
Dendritic cells play a critical role in the activation of CD4 T cells because of their crucial role in antigen presentation to T cells. To carry out antigen presentation, dendritic cells need to express costimulatory molecules to induce efficient T-cell responses. One such costimulatory molecule is CD70, which is the only known ligand for the receptor CD27.18 Here, it is demonstrated that the expression of CD70 on human PBMC-derived dendritic cells is upregulated as the concentration of glucose used to prime these cells was increased. Although the expression and function of CD70 on human immune cells are not fully understood yet, the results are in accordance with others highlighting that CD27-CD70 plays a crucial role in the adaptive immune response in humans.19,20 However, this study is the first to acknowledge looking at the CD27 and CD70 profiles on immune cells in a model of hyperglycemia. In a study using an adenovirus infection model,20 it was found that regardless of the mechanism by which CD70 was induced on activated dendritic cells, a blockade of CD70 on the activated dendritic cells significantly reduced their immunogenicity. Additionally, in a mouse model of colitis, anti-CD70 antibody was found to be effective in the prevention and reversal of disease caused by the significant reduction in IFN-γ produced by dendritic cells. Results from this study’s model show dendritic cells as the primary producers of IFN-γ, highlighting an important pathway in type 2 diabetes or prediabetes that could be explored for future therapies. Furthermore, CD27 function and eventual downregulation on T cells only happens through interaction with CD70. This means that the inflammatory response because of CD27-CD70 signaling would fail to occur in the absence of CD70. Recent evidence points to chronic and low-grade inflammation as the major driving force for the pathogenesis of prediabetes into type 2 diabetes with immune cells, such as APCs, T cells, and B cells, as the key players in inflammation.21,22 This study suggests that CD27-CD70 signaling is one pathway by which immune cells become activated in hyperglycemic conditions and proposes future studies to investigate the use of targeted CD70 therapy to control and selectively reduce the chronic inflammation driving the pathogenesis of diabetes. These data also show that the dendritic cells are the predominant cells expressing CD70 in the study’s model of hyperglycemia. Therefore, this selective targeting of dendritic cells would potentially avoid generalized immunosuppression. Although the expression of CD27 on B cells has not been investigated in this study’s model, it is an area that warrants future research. Additionally, CD27 could be analyzed to predict progression of prediabetes to type 2 diabetes or to detect the subclinical inflammatory processes that drive its progression and risks for comorbidities, perhaps when analyzed together with CD11a, per the previously published results.4 Because most clinical laboratories contain a flow cytometer, even a basic 3–4 monoclonal antibody panel could detect the CD27 and CD11a expression on a patient’s T cells to determine the proportion of naïve versus effector T cells present. Taken in context with the diagnosis of prediabetes or diabetes in a patient, this could help guide subsequent therapeutic options.
Patients with diabetes often contract diseases and infections at a higher frequency than people without diabetes.23,24 However, the underlying mechanism behind this process and why this occurs in a state of chronic inflammation are currently unknown. A recent study found that patients with type 2 diabetes had a higher frequency of B cells and effector T cells as well as a normal cytokine profile from myeloid and NK cells.25 Although this study focuses exclusively on CD4 T cells and CD11+ PBMC-derived dendritic cells, it is true that the phenotypic and functional capacity of both dendritic cells and CD4 T cells is not only preserved but also increased with elevated glucose concentrations. Interestingly, the CD4 T cells in this study’s model had an increased effector phenotype in terms of mobilization and costimulation but did not produce the inflammatory cytokines IFN-γ and TNF-α after 7 days in coculture with dendritic cells. An important aspect to note is that this study only looked at 1 time point (7 days) after coculture and priming of the CD4 T cells. In a study investigating the role of CD70 in human monocyte-derived dendritic cells to be used in tumor immunotherapy, investigators showed an increase in cytokine production from CD4 T cells after being in coculture with dendritic cells for 8 days. This was dependent on IFN-α being present in the priming conditions for the dendritic cells.26 Therefore, it is possible that if this study looked at other time points in the coculture system or used a different method of stimulation, CD4 T-cell cytokine production would be observed. Nonetheless, this study recognizes that it may seem counterintuitive for a person with type 2 diabetes to have an increased effector T-cell population and activated dendritic cells yet still be more prone to infections than an individual without diabetes. However, this work corroborates previously published studies on the immune profile of individuals with diabetes. It should be noted that there are many other aspects of the immune system that could be defective in people with type 2 diabetes. For example, the innate arm and the cells/defenses it comprises could have significant defects in people with type 2 diabetes that are yet to be discovered.
In conclusion, this study has shown that dendritic cells matured in hyperglycemic conditions display an activated phenotype, confirming a recent study.27 Additionally, the study demonstrated that activated dendritic cells alter the phenotype and function of the CD4 T cells they subsequently prime. This supports the notion that immune cells play a crucial role in chronic inflammation observed in patients with type 2 diabetes or prediabetes. The CD27-CD70 costimulatory pair has been linked to inflammation in a variety of different autoimmune and chronic inflammatory conditions.28⇓⇓-31 The results presented in this study provide novel evidence that the CD27-CD70 axis is activated in diabetes or hyperglycemic conditions and support future examination of cell surface markers, particularly CD27 and other CD4 T-cell markers of effector function, to further stratify patients with prediabetes or diabetes by level of chronic inflammation and identify patients most at risk of comorbidities. Collectively, results reported from this study lend novel insight into the role that immune cells play in hyperglycemia and diabetes.
FINANCIAL SUPPORT
This work was supported by an American Society of Clinical Laboratory Science Education and a research fund grant in 2019 (awarded to NML).
- Received September 4, 2019.
- Accepted December 14, 2019.
American Society for Clinical Laboratory Science