This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Address for Correspondence: Brooke Dubansky
, Louisiana State University School of Veterinary Medicine, bdubansky{at}lsu.edu
LEARNING OBJECTIVES
1. Describe typical histological characteristics of dysplasia in colorectal lesions.
2. Discuss the generalized progression of adenocarcinoma from a benign adenoma (polyp).
3. List and describe some general histological characteristics of the most common subtypes of adenomas and adenocarcinomas.
ABSTRACT
Classification and staging of recognized colorectal cancer subtypes are based on histological characteristics of the lesion and predictable changes in morphology. Adenocarcinoma arising from adenomatous polyps is the most common type of colorectal cancer. The histological progression from benign, non-neoplastic polyps to invasive neoplastic adenocarcinoma is, in many cases, correlated with specific molecular aberrations. This review describes the classification, histological characteristics, and progression of non-neoplastic, as well as benign and malignant neoplastic, colorectal lesions.
- CRC - colorectal cancer
- LCRC - left-sided colorectal cancer
- MSI - microsatellite instability
- RCRC - right-sided colorectal cancer
- WHO - World Health Organization
INTRODUCTION
According to the American Cancer Society, colorectal cancer (CRC) is the third most common cancer diagnosed in men and women in the United States, with 106 180 new cases of colon cancer and 44 850 new cases of rectal cancer predicted in 2022. Signs and symptoms of CRC include a change in bowel habits, constipation (in some cases diarrhea), rectal bleeding or blood in the stool, abdominal discomfort, and unexplained weight loss. As most CRCs begin as a polyp, early detection and removal via colonoscopy are key to diagnosis and treatment and improving the prognosis. The purpose of this article is to review the classification and histological characteristics of various CRC subtypes, with emphasis on the adenocarcinomas, which make up the majority of CRC cases. Subsequent articles in this focus series build upon these basic histological classifications. Normal histology of the colon is reviewed when appropriate; however, a more in-depth review of normal colon histology is beyond the scope of this article, and the reader is referred to a textbook of histology.1
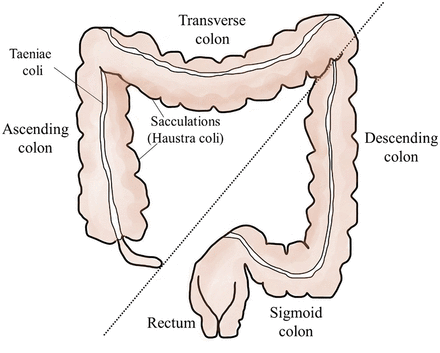
The colon is divided anatomically into ascending, transverse, descending, and sigmoid sections (Figure 1). The sigmoid colon terminates in the rectum, which exits the body at the anus. Outer longitudinal bands of smooth muscle called taeniae coli span the length of the colon and contract to move fecal material through the colon. Taeniae coli form sacculations, also called haustra coli, which compartmentalize and partially control the movement of fecal material through the large intestine.1 The colon and rectal segments of the large intestine are histologically similar, with a mucosal layer specialized for protection and absorption of water.1 The underlying lamina propria, unlike in the small intestine, lacks lymphatic vessels.1 This is an important modification, as invasive CRCs are slower to metastasize since they must penetrate the deeper tissues of the intestinal wall to access areas with lymphatic drainage.1
The large intestine (colon and rectum). The colon is divided anatomically into ascending, transverse, descending, and sigmoid sections. The sigmoid colon terminates in the rectum, which exits the body at the anus. Right-sided colorectal cancers (RCRCs) involve the ascending and transverse colon (left of dotted line); left-sided colorectal cancers (LCRCs) involve the descending and sigmoid colon as well as the rectum.
The regional location of the initial neoplastic lesion (polyp) in the large intestine is correlated with the progression of the disease and, in some cases, the outcome.2 For prognostication, CRC is described as affecting 2 sectors: 1) the right side as right-sided CRC (RCRC), involving the ascending and transverse colon; and 2) the left side as left-sided CRC (LCRC), involving the descending and sigmoid colon as well as the rectum.2 RCRC incidence is lower than LCRC, although the incidence of this type is increasing, especially in females.2,3 Patients with RCRC typically present with a higher grade and stage of cancer, with larger tumors of the mucinous subtype.2 These tumors typically have a worse overall survival rate than LCRC, which is characterized by smaller, lower-stage tumors.2 Other characteristics such as clinical presentation, immunologic response, and the specific molecular aberrations of CRCs can also be correlated to this regional variation.2
PROGRESSION OF COLORECTAL LESIONS (BENIGN TO MALIGNANT)
Non-neoplastic lesions in the colon include inflammatory bowel disease (ulcerative colitis) and Crohn’s disease. Benign, neoplastic lesions are called polyps.4 With accumulating mutations, benign polyps can progress to cancerous lesions. Adenocarcinomas, which form most CRCs, are one such lesion that can progress from inflammatory bowel disease (nonpolyploid) or benign polyps (polyploid).4 Additional carcinomas frequently seen and differentiated based on histologic appearance include adenosquamous, spindle cell, and squamous cell carcinomas. Undifferentiated carcinomas can also occur. The increased risk of cancerous transformation of inflammatory bowel diseases, such as Crohn’s and ulcerative colitis, is an important reason to screen affected patients frequently for dysplastic change.4 A dysplastic response precedes most cancerous CRC lesions and occurs when the epithelial lining of the colon no longer exhibits normal histological features, often due to recurrent cycles of inflammation and healing.4,5
Rather than a distinct separation between benign and malignant colorectal neoplasms, it is perhaps best to view the progression of the disease on a continuum. Normal colon glands are single, tubular invaginations of the epithelium lined by a single layer of columnar cells that are interspersed with numerous mucin-secreting goblet cells.1 These features, along with a thickened lamina propria and a robust population of immune cells, facilitate absorption and transport of water and electrolytes and protect the underlying tissue layers from bacterial invasion.1 Dysplastic change may result from a single mutation in one of the epithelial cells, which prevents it from adhering to normal growth regulations. Dysplastic epithelium in the colon and rectum often becomes stratified, and nuclear changes include hyperchromasia and elongation.4⇓-6 Low-grade dysplasia is characterized by retention of recognizable gland architecture and fewer epithelial abnormalities than high-grade dysplasia, which is characterized by more severe cell and nuclear abnormalities and a loss of gland architecture.5 Low-grade dysplasia carries less risk of cancerous progression than high-grade dysplasia. The dysplastic response may be reversible, depending on the underlying cause of the transformation and whether the stressors are relieved.
Additional mutations in the rapidly dividing cells of dysplastic epithelium may lead to a loss of normal gland architecture, resulting in an overgrowth of epithelium, which can lead to polyp formation. At this point the growth is referred to as an in situ neoplasm, provided it has not invaded beyond the lamina propria into the underlying submucosa where it may access lymphatic vessels.4,5 Additional mutations may lead to malignancy, characterized by invasion of the submucosa, and even the external muscular layers (muscularis externa). Lymphovascular invasion, lymph node involvement, and metastasis are characteristics of advanced neoplastic disease.4,5
STAGING OF CRC
The stages of CRC are based on the progressive continuum described earlier. Staging involves the histologic appearance of the tumor as well as other characteristics of invasion and metastasis. The earliest stage, sometime called stage 0, describes a carcinoma in situ, which is confined to the epithelium or lamina propria. Stage I is characterized by invasion into and beyond the submucosal layers where tumor cells may gain access to the lymphovascular system.4 Stages 2–3 exhibit progressive characteristics, including deeper invasion, lymphovascular invasion, and degrees of lymph node involvement. Stage IV, the last stage, is characterized by distant metastasis to organs and tissue, such as the liver, lung, or peritoneum. The scoring system for staging CRCs uses the TNM scale, which scores tumor characteristics (T) (grade, size), nodal involvement (N), and metastasis (M) to distant organs.4 Regardless of the T and N scores, any distant metastasis results in a stage IV diagnosis.
CLASSIFICATION AND CHARACTERISTICS OF BENIGN POLYPS
Raised, benign colorectal lesions (polyps) may be inflammatory, hamartomatous, hyperplastic, or adenomatous (Table 1).6 A fifth category of benign colorectal lesion is the sessile serrated lesion (formerly called sessile serrated polyp or adenoma),7,8 which is an intermediate subtype between hyperplastic and adenomatous polyps and is characterized by a particular developmental pathway that results in a distinct histological appearance (Table 1).4 Macroscopically, polyps may be pedunculated (containing a broad body and small, thin stalk) or sessile (flattened).4 Adenomatous polyps exhibit a glandular architecture and are the type that most frequently progresses to adenocarcinoma, the most common type of CRC.4,5
Basic characteristics of benign colorectal polyps
There are 3 types of adenomatous polyp that are characterized by the histology of their gland structure: tubular adenomas, villous adenomas, and tubulovillous adenomas. Tubular adenomas are the most common subtype and comprise about 75%–80% of benign adenomatous polyps. Other adenomas are represented by villous and tubulovillous subtypes.6,9 While making up fewer of the adenoma subtypes, villous and tubulovillous adenomas are more likely to undergo malignant transformation than tubular adenomas.9
Tubular adenomatous polyps are typically small (<10 mm in diameter), pedunculated, and contain numerous elongated branching crypts lined by a mucus-secreting dysplastic epithelium.6 The size of the polyp may be proportional to the likelihood of malignant transformation.4,6,9 Villous adenomatous polyps are usually large (>20 mm in diameter) and sessile and contain elongated villi in a papillary growth pattern, giving them a velvety appearance.6 The dysplastic glandular epithelium often lacks mucous-secreting cells. Tubulovillous adenomatous polyps contain intermediate features of the previously described morphologies in different regions of the polyp.4,6
Sessile serrated lesions, which are histologically intermediate between hyperplastic polyps and adenomatous polyps described above, are characterized by low- to high-grade dysplasia, a serrated glandular luminal profile, and an increased risk of malignant transformation.3,4 One study found that around 75% of serrated adenomas carry a BRAF mutation.10 Serrated adenomas represent a distinct malignant pathway to adenocarcinoma.9
CLASSIFICATION AND CHARACTERISTICS OF CRC
The World Health Organization’s (WHO’s) classification of colorectal carcinomas identifies 5 categories: 1) adenocarcinoma, 2) adenosquamous carcinoma, 3) spindle cell carcinoma, 4) squamous cell carcinoma, and 5) undifferentiated carcinoma. Adenocarcinomas are the most common type, making up about 90% of all colorectal carcinomas,4,5,7,11 and are the only type discussed in detail in this review. Adenocarcinomas commonly arise from benign adenomatous polyps and progress to invasive (malignant) neoplasms. Histological grading of adenocarcinomas is independent of staging and may help to determine prognosis in conventional adenocarcinomas.5 As defined by Fleming et al, well-differentiated adenocarcinomas (low grade) contain numerous recognizable epithelial glands, constituting greater than 95% of the tumor, while moderately differentiated adenocarcinomas, which comprise most cases at diagnosis, exhibit 50%–90% recognizable gland formation.5 Poorly differentiated adenocarcinomas (high grade) show less than 50% gland formation. In less common adenocarcinoma subtypes (described later), histological grade may be less likely to predict patient outcome than molecular characteristics.5
To become invasive, adenocarcinomas must penetrate through the basement membrane, underlying connective tissue (lamina propria), and muscularis mucosa into the submucosa. Invasive tumors that only invade into the lamina propria are not likely to metastasize because the lamina propria lacks sufficient lymphatic vasculature.1,5 Once the tumor invades into the lymphovascular network of the submucosa, the most common site of metastasis for most subtypes is the liver. Mucinous subtypes, however, exhibit an uncommon metastatic pattern to the surface of the peritoneal cavity.11,12 On histology, the invasive adenocarcinoma may appear sessile or pedunculated, with a disruption in the muscularis mucosa, indicating movement into the submucosa. Lymphovascular invasion indicates a higher-stage tumor and suggests an increased probability of metastasis. The degree of sentinel and distant lymph node involvement is also important in the development of the staging score.4 Desmoplasia (a fibroproliferative response of the tumor stroma) and dirty necrosis (necrotic debris in the gland lumina) are unique histological features of adenocarcinomas.4,5
ADENOCARCINOMA SUBTYPES
The WHO recognizes 6 subtypes of adenocarcinoma based on histological appearance: 1) cribriform-comedo, 2) medullary, 3) micropapillary, 4) mucinous, 5) serrated, and 6) signet ring (Table 2). Mucinous subtypes make up approximately 10% of adenocarcinomas.12 Mucinous subtype tumors are characterized by the excessive amount of mucin composing the tumor, occasionally approaching half of the total tumor volume.5,13 Cells with a signet ring morphology and intracellular mucin may make up less than 50% of the tumor.5 Mucinous carcinomas are more commonly RCRCs and generally present a high stage at diagnosis.13 These tumors are less responsive to treatment compared to conventional adenocarcinoma, and, as mentioned previously, these tumors frequently metastasize to the peritoneum rather than to the liver.12 Molecular characteristics of mucinous adenocarcinoma include microsatellite instability (MSI) (discussed in subsequent sections) and a higher rate of KRAS, BRAF, and PI3K mutations.12,13 Mucinous adenocarcinoma is often associated with a hereditary predisposition for cancer called Lynch syndrome.5,12
Colorectal cancer subtypes
The signet ring subtype of adenocarcinoma is rare, making up less than 1% of adenocarcinomas, and is histologically related to the mucinous subtype described above.5,12,14 Mucin makes up less than 50% of the tumor volume and is associated with RCRC, MSI, and BRAF and KRAS mutations.12 The overall prognosis is poor, usually because of the high stage at diagnosis, with a mean 5-year survival rate of about 30%.12,14 Unlike many of the other CRCs, the signet ring adenocarcinoma tends to affect patients under 60 years of age.12 The tumor derives its name from the appearance of the tumor cell, which contains a large mucinous vacuole that displaces the nucleus to the outer rim of the cell membrane, thus giving it the appearance of a signet ring.5
Another rare subtype, medullary adenocarcinoma, formerly known as large cell adenocarcinoma with minimal differentiation, is difficult to diagnose because of its poor histological differentiation.12,15 This subtype occurs most commonly on the right side of the colon (RCRC) and is characterized by solid sheets of tumor cells and a prominent lymphocytic infiltrate.5,11,12,15 Despite being poorly differentiated, medullary adenocarcinoma carries a good prognosis, as it is less likely to spread to lymph nodes.5,11,12,15 This subtype is strongly associated with MSI and BRAF mutations.5,11,12,15
Serrated adenocarcinoma may be mucinous or nonmucinous and is characterized by the saw-toothed pattern of the surface of the gland epithelium. This pattern is induced by stages of epithelial growth separated by resting stages associated with a lack of apoptotic activity.5 Like serrated adenoma, serrated adenocarcinoma is histologically similar to hyperplastic polyps.13 Up to half of all cases develop from benign serrated adenoma and affect the right side of the colon (RCRC).13 This subtype is associated with BRAF and KRAS mutations and carries a poor prognosis.13
Micropapillary adenocarcinoma in the colon, as in other organs, shows distinct micropapillary projections within lacunae, which is a histological feature that can mimic lymphovascular invasion (identified by clusters of tumor cells floating in vessels).12,16 The epithelium of the micropapillae exhibit reverse polarity, with basal and apical protein expression reversed, which is a unique characteristic of this subtype.16 Micropapillary adenocarcinoma carries a poor prognosis because lymphovascular invasion, lymph node involvement, and metastasis are common features.12,16
Cribriform adenocarcinoma in the colon presents with circular nests of epithelioid tumor cells, often with dirty necrosis at the center.17,18 This histological subtype resembles cribriform subtypes of other cancers.17,18 Epithelial nests are compartmentalized by an extensive fibrous stroma. This subtype is associated with MSI, a high rate of lymphovascular invasion, nodal involvement, and metastasis. Hence, it exhibits a poor 5-year survival rate.18
CONCLUSION
Adenocarcinoma, being the most common type of CRC, exhibits a large variety of histological characteristics and subtypes. The histological features of adenocarcinoma in general determine tumor grade and subtype classification but are not always reflective of prognosis. Common molecular aberrations that drive the progression of adenocarcinoma include KRAS and BRAF mutations and MSI, among others. In the context of tumor histology, molecular variations may be more important for assessing tumor aggression and patient outcome.
- Received February 29, 2024.
- Accepted April 20, 2024.
American Society for Clinical Laboratory Science