This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
- Weber State University
- Weber State University
- Weber State University
- Weber State University
- Weber State University
- Weber State University
- Weber State University
- Address for Correspondence: Katie Wilkinson
, Weber State University, katie.a.wilkinson{at}dmu.edu
ABSTRACT
Transition metal inorganic compounds, also known as polyoxometalates (POM), have many biological applications, such as antiviral, antitumor, and antibacterial therapies. The objective of this study was to determine if the POM α-K6P2W18O62 · 14H2O could overcome the resistance of methicillin-resistant Staphylococcus aureus (MRSA). To determine the minimal inhibitory concentration (MIC) and possible synergistic effects, multiple dilutions of oxacillin and POM were combined with inoculums of MRSA. Susceptibility (MIC ≤ 0.25 µg/mL oxacillin) was achieved at a concentration of 5 µM of α-K6P2W18O62 · 14H2O. The POM enhanced the efficacy of oxacillin and additionally showed low toxicity to mammalian cell cultures in vitro. The effect of the POM on mecA gene transcription was assessed using reverse transcriptase–quantitative polymerase chain reaction and showed a reduction in messenger RNA transcription at effective POM doses; however, increased transcription was observed at higher POM oxacillin doses. This pilot study illustrates that α-K6P2W18O62 · 14H2O could be used in conjunction with oxacillin. However, further testing needs to be completed.
- ATCC - American Type Culture Collection
- cDNA - complementary DNA
- HCT116 - human colorectal carcinoma cells
- LC50 - lethal concentration 50
- MBC - minimum bactericidal concentration
- mRNA - messenger RNA
- MRSA - methicillin-resistant Staphylococcus aureus
- MSSA - methicillin-susceptible Staphylococcus aureus
- MIC - minimal inhibitory concentration
- PBP2A - alternative penicillin binding protein
- POM - polyoxometalate
- rRNA - ribosomal RNA
- RT-PCR - reverse transcriptase–polymerase chain reaction
- RT-qPCR - reverse transcriptase–quantitative polymerase reaction
- SBA - sheep’s blood agar
- W - tungsten
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is caused by a variety of mechanisms. MRSA is capable of evading β-lactam antibiotics by genetic alterations, including the mecC and mecA genes.1 This study focused on the mecA gene, which codes for an alternative penicillin binding protein (PBP2A). β-lactam antibiotics, such as oxacillin, are unable to efficiently bind with this alternative form of PBP2A and are unable to act as cell wall synthesis inhibitors.2 MRSA’s resistance to antibiotics leaves health care professionals with limited treatment options. Patients infected with MRSA have an average hospital stay 2.6 days longer than patients with a methicillin-susceptible Staphylococcus aureus (MSSA) infection, and an average cost of $13,901 more than MSSA patients.3 Because of the increase of asymptomatic carrier cases, MRSA is one of the leading causes of hospital-acquired infections. Therefore, hospitals are enforcing presurgical screenings for MRSA to try to decrease the chance of transmission to other patients. Two methods of presurgical screenings are used. Once a patient has positive results on screening, they are prescribed immediate isolation precautions.4
Polyoxometalates (POMs) are inorganic compounds that contain a transition metal and have been studied for a variety of uses. In high concentrations, they induce apoptosis in some types of cancer cells. They also have an inhibitory effect on some viral infections by disrupting a step between attachment and penetration.5,6 While transition metals are notorious for being toxic in high doses, tungsten (W) has been shown to be relatively nontoxic compared with other transition metals, such as mercury. There are many hypotheses as to why W-containing compounds are not as toxic. The most probable theory is that when W is in an oxoanionic state, it is water soluble.7
This study analyzed the effect of the POM α-K6P2W18O62 and oxacillin against MRSA. The POM was also tested against mammalian cells to determine the lethal concentration that was required to kill 50% of the cell population (lethal concentration 50 or LC50). It was discovered that the POM was effective at overcoming the methicillin resistance in MRSA and showed a low toxicity at bio-reactive concentrations. To determine if the POM influenced the presence of mecA, messenger RNA (mRNA) levels of mecA were measured using reverse transcriptase–quantitative polymerase chain reaction (RT-qPCR). In previous research, reverse transcriptase–polymerase chain reaction (RT-PCR) was used, and it was suggested that POMs were overcoming resistance by decreasing the expression of mecA.2 In this study, a more sensitive and accurate method, RT-qPCR, was used. α-K6P2W18O62 was found to decrease mRNA expression at low concentrations, yet it increased mRNA expression at higher concentrations. This is in contrast to previous research and may suggest an alternative effect on the mecA gene: perhaps a posttranslational modification.
MATERIALS AND METHODS
α-K6P2W18O62 Synthesis
α-K6P2W18O62 · 14H2O was synthesized according to procedures in a previously published work.8 While stirring vigorously and insulating the system with warm water, 250 mL of 4M HCl was slowly added to 301.7 g of Na2WO4· 2H2O (formula weight 329.87) until the solution became limpid. Then, 250 mL of 4M H3PO4 (aqueous) was added slowly with an addition funnel to the previous solution, still stirring vigorously. The product was then refluxed under positive pressure of argon gas for 24 hours. The solution changed from colorless to a light green. One-hundred and fifty grams of potassium chloride powder was added to the refluxed solution, and a white precipitate formed. This precipitate was then filtered and air dried by aspiration. Once the solution was completely dry, 650 mL of distilled H2O was added. A limpid solution was obtained after 5 days of stirring continuously at room temperature. After the solution was dissolved and colorless, it was then allowed to reflux at 80 °C for 3 days and then cooled to room temperature. Once at room temperature, the solution was placed on ice for 72 hours and green crystals were produced. Proper structure of the compound was confirmed by comparing infrared spectroscopy and phosphorus (31P) nuclear magnetic resonance spectroscopy to previously published work.8 Confirmation was determined by the correct phosphorus-oxygen stretching and vibration peak at 1,086 cm-1 and 957 cm-1, and by its 31P chemical shift at -13.577 ppm, which is in the delta range when compared to referenced literature.8 While the structural confirmation was performed, the stability of the POM over time was not tested.
Polyoxometalates Susceptibility Testing
To determine if the POM alone could inhibit bacterial growth, a minimum bactericidal concentration (MBC) and a minimal inhibitory concentration (MIC) of the POM was established for MRSA (American Type Culture Collection [ATCC] 43300). Using a concentrated stock solution of the POM at 20,000 µM, 1:2 dilutions were made with 1 mL of Columbia Broth growth media down to 39 µM concentration. Then, 4 µL of a MRSA suspension was added to obtain a 0.5 McFarland standard concentration in each dilution tube. After 24 hours of incubation at 37 °C, the tubes were recorded for growth or no growth based on turbidity. Tubes that demonstrated no visible growth had a sample taken that was then plated onto sheep’s blood agar (SBA) media. These plates were then incubated at 37 °C for an additional 24 hours, then evaluated for colony growth.
Minimal Inhibitory Concentration Panel Synergy Testing
Dilutions of 5,000 µM, 500 µM, 50 µM, and 5 µM of α-K6P2W18O62 · 14H2O were tested in combination with preset concentrations of oxacillin on the MIC panels. These concentrations ranged from 0.25–4 µg/mL. Two sets of experiments were performed. The first was termed coincubation. MicroScan™ MIC panels were inoculated with MRSA and various concentrations of POM. This was done according to Clinical and Laboratory Standard Institute protocols and the manufacturer’s guidelines.9 The MIC plates were then incubated for 24 hours at 37 °C in an air incubator. The susceptibility results were read on a Beckman Coulter MicroScan Walkaway™ instrument. In a second set of experiments, termed preincubation, the POM was allowed to incubate with MRSA before inoculation of the MIC panel. This was achieved by inoculating 3 mL of Columbia broth with MRSA and various concentrations of POM. They incubated overnight in an air incubator at 37 °C while being agitated on a rocker. The dilutions were then prepared to a 0.5 McFarland standard, inoculated in the MIC panel, and incubated for an additional 24 hours before being read on the Beckman Coulter Walkway™. Each combination of POM was run in triplicate. When the POM was added to the inoculums of saline and oxacillin, a blue color was observed. To ensure the POM did not interfere with the reading performance of the instrument, each MIC was confirmed by hand reading the panels.
Toxicity Study
Human colorectal carcinoma cells (HCT116) were obtained from ATCC as a model for mammalian cell cytotoxicity. The cells were grown in Dulbecco’s Modified Eagle Medium in the presence of α-K6P2W18O62 · 14H2O at 0.5 µM, 5 µM, 50 µM, and 500 µM for 72 hours. The cells were then fixed with ice-cold methanol and stained with crystal violet (0.5% in 20% MeOH/H2O). To quantitate the stain, a 2% sodium dodecyl sulfate solution was used to extract the crystal violet stain from the fixed cells and then analyzed at 595 nm on a Tecan UV-Vis™ spectrometer. Dilutions were run in duplicate, and an average of the absorbance reading was reported.
Real-Time Quantitative Polymerase Chain Reaction
Six separate samples of MRSA (ATCC 43300) were cultured in tryptic soy broth for 2.5 hours at 37 °C. Sample 1 included MRSA; sample 2 included MRSA with 4 µg/mL oxacillin; sample 3 included MRSA with 5 µM α-K6P2W18O62 · 14H2O; sample 4 included MRSA with 5 µM α-K6P2W18O62 · 14H2O and 4 µg/mL oxacillin; and sample 5 included MRSA with 50 µM α-K6P2W18O62 · 14H2O, sample 6 included MRSA with 4 µg/mL oxacillin and 50 µM α-K6P2W18O62 · 14H2O. Following incubation, total RNA extraction was performed via TRIzol™ Max™ Bacterial RNA Isolation Kit by Ambion per manufacturer’s guidelines. Each sample was then deoxyribonucleased to remove any residual DNA. Complementary DNA (cDNA) libraries were generated from RNA extracts using Moloney murine leukemia virus reverse transcriptase by Invitrogen per manufacturer’s protocol with gene-specific reverse primers for 16s ribosomal RNA (rRNA) and mecA. Nucleic acid quantitation and sample purity was performed using standard absorbance at 260/280 nm using an EPOCH-2 microplate reader from Biotek™. RT-qPCR was performed on a QuantStudio 3™ Real-Time PCR instrument from Applied Biosystems. The reaction mixture consisted of PowerUP™ SYBR™ Green Master Mix, 300 nM of each primer, and 1uL cDNA. The mecA primers (F: 5′-ATCCACCCTCAAACAGGTGAAT-3′, R: 5′GGAACTTGTTGAGCAGAGGTTC-3′) were designed using the Benchling software with a product size of 139 base pairs.10 The 16s rRNA primers (F: 5′-CGTGCCTAATACATGCAAGTC-3′, R: 5′-CCGTCTTTCACTTTTGAACCA-3′) were designed per a previous publication.2 A standard curve was created using cDNA from a MRSA isolate in a 5.10-fold dilution for both 16s rRNA and mecA. Qualitative polymerase chain reaction was performed with the following conditions: 50 °C for 2 minutes, 95 °C for 2 minutes, and then 40 cycles of 95 °C for 15 seconds denaturation, 61 °C (mecA) or 59 °C (16s) annealing for 15 seconds, and 72 °C extension for 1 minute. A melt curve was also performed to assess specificity. Relative quantification fold changes were calculated in the QuantStudio Design and Analysis software with the standard curve method that uses 16s rRNA as the endogenous control and sample 2 as the reference sample.
RESULTS
Susceptibility Testing
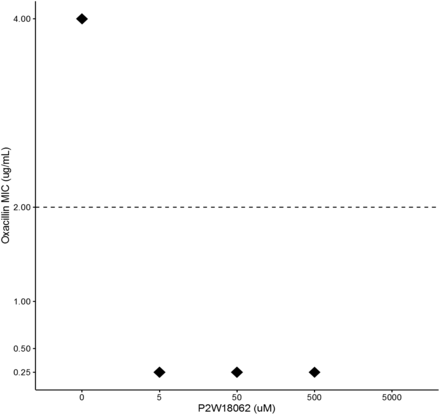
To determine if the POM alone could inhibit the growth of MRSA, an MBC and MIC was established. When observing the tubes for growth, turbidity was noticed from 39 to 5,000 µM. A sample from all tubes presenting with no turbidity was then placed onto SBA media. Colony growth was not observed on any of the SBA plates, which indicated that the POM alone can inhibit the growth of MRSA at high concentrations. The result was MIC and MBC of the POM at 10,000 µM. Two experiments were completed to establish any synergistic effects of POM and oxacillin; these were termed coincubation and preincubation. The coincubation study revealed that the POM and oxacillin resulted in MRSA being susceptible to oxacillin at 5 µM (Figure 1). However, the preincubation study of POM and oxacillin did not reach susceptibility until 500 µM. These results showed that the POM was most effective when coincubated with oxacillin and MRSA in inducing susceptibility.
Coincubation effect of α-K6P2W18O62 · 14H2O on MRSA MIC of MRSA in combination with oxacillin (ug/mL) and 3 different concentrations of α-K6P2W18O62 · 14H2O (uM) prepared by coincubation techniques. Each concentration was run in triplicate and obtained the same MIC value, which are represented by the diamonds. The dashed line represents the oxacillin breakpoint at which Staphylococcus aureus is considered resistant or susceptible.
α-K6P2W18O62 · 14H2O Toxicity
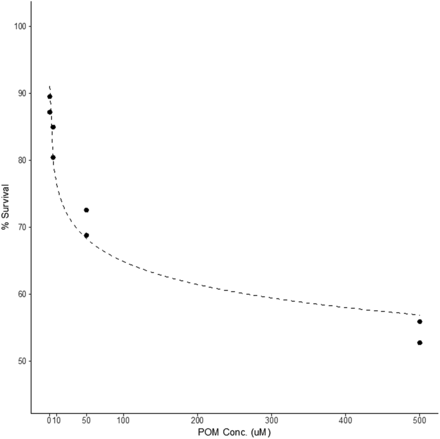
To assess the viability of using α-K6P2W18O62 · 14H2O as a treatment option, toxicity studies against HCT116 cancer cells revealed that α-K6P2W18O62 · 14H2O is not effective in killing mammalian cells, as the LC50 was not reached at concentrations as high as 500 µM (Figure 2). In POM concentrations that were proven effective 80% to 90% of the cells survived. The results indicate that the POM appears to have a low toxicity.
Toxicity of α-K6P2W18O62 · 14H2O on HCT116 cells. Points represent percentage of cell survival for 5 concentrations of POM (0.5,5, 50 and 500uM). Each concentration was run in duplicate and compared with control samples to calculate the percentage of survival. The dashed line represents a fitted logarithmic curve.
mecA Messenger RNA Expression
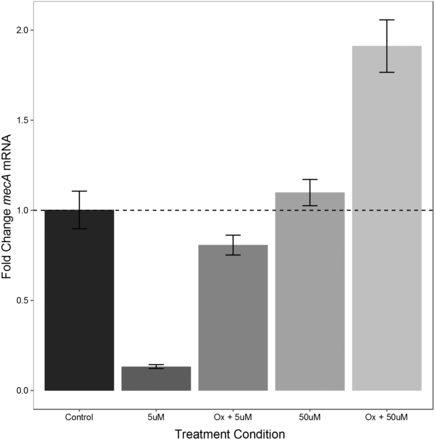
Previous work has shown an effect of other POMs on the mecA gene expression in MRSA using RT-PCR products visualized on an electrophoresis gel.2 In this study, a more sensitive and accurate approach of RT-qPCR was used. The results of the RT-qPCR are shown in Figure 3. There was an average of 86.7% reduction (0.133 average fold decrease) in mecA mRNA expression when MRSA was incubated with α-K6P2W18O62 · 14H2O at a 5 µM concentration and a 20% reduction (0.807-fold average decrease) when incubated with 5 µM of α-K6P2W18O62 · 14H2O and 4 µg/mL of oxacillin. The 20% reduction correlated with a susceptible phenotype in the MIC testing. Yet, surprisingly, there was an observed increase in mecA mRNA production in both samples containing 50 µM of α-K6P2W18O62 · 14H2O alone or in combination with 4 µg/mL of oxacillin (1.099 and 1.91 average fold increase respectively) even though this higher concentration of POM was also associated with a susceptible phenotype. These results taken together would suggest that α-K6P2W18O62 · 14H2O does not act directly against mRNA production as previous studies suggest.
The effects of α-K6P2W18O62 · 14H2O on mecA expression MRSA. Overnight cultures of MRSA were incubated for 3 hours at 37 °C without α-K6P2W18O62 · 14H2O in the presence of 4 µg/mL oxacillin to induce mecA expression [control], α-K6P2W18O62 · 14H2O alone [5uM and 50uM], and combinations of 4 ug/mL oxacillin and α-K6P2W18O62 · 14H2O [Ox + 5uM, Ox + 50uM]. Data represent mean fold changes (error bars = +/- 2 SEM) in mecA expression determined by RT-qPCR. All fold changes values were calculated using a standard curve method using the 16s rRNA gene as the endogenous control and the control sample as the reference.
DISCUSSION
With MRSA having an altered PBP2A, the ability to be resistant to β-lactam antibiotics such as oxacillin has left health care providers with limited treatment options. POM has been used against viruses and tumors at high concentrations. This study analyzed the effect of the POM α-K6P2W18O62 · 14H2O in combination with oxacillin against MRSA. It was proven that a combination of α-K6P2W18O62 · 14H2O with oxacillin is effective at inhibiting MRSA in ATCC 43300. Coincubation of α-K6P2W18O62 · 14H2O with oxacillin made MRSA susceptible with as little as 5 µM of POM, while preincubation was not able to obtain susceptibility until 500 µM. The toxicity study revealed that at effective doses, the POM did not reach the LC50. Previous research has used different POMs to try and produce a synergistic effect; they were able to do so at high concentrations. K6P2W18O62 · 14H2O was able to do it at 5 µM. α-K6P2W18O62 was found to decrease mRNA expression at low concentrations yet increase the expression at higher concentration, which is counter to previous research and may suggest an alternative effect on the mecA gene. The use of the RT-qPCR method using endogenous controls and the standard curve method is more accurate and sensitive of a methodology compared with conventional RT-PCR and the comparison of electrophoresis band intensity. This research is an important pilot study because it illustrates that K6P2W18O62 · 14H2O and oxacillin are capable of antimicrobial activity in the laboratory strain ATCC 43300.
While this study was carefully designed, some limitations were obtained. This was a pilot study; therefore, the laboratory strain ATCC 43300 of MRSA was the only strain used for analysis. Other laboratory strains of MRSA, and clinical strains of MRSA would need to be obtained and tested in the same manner prior to any animal models being studied. If other strains of MRSA had the same conclusion as ATCC 4300, animal models with active MRSA infections would then be studied for routes of administering α-K6P2W18O62 · 14H2O. These studies may include effectiveness of taking the POM orally, intravenously, and topically. While the MIC panels contain an abundance of antibiotics, oxacillin was the only one analyzed in this study. Other penicillins would need testing in the same manner as oxacillin. The POM structure was confirmed; however, the stability over long periods of time was not determined. To determine stability, a forced-stability, accelerated-stability, and real-time study may be performed. This would be accomplished by subjecting the POM to different temperatures, humidity, light, and time periods. Analysis of the product would then be conducted by liquid chromatography and mass spectrometry. The analysis would help determine proper storage conditions, as well as shelf life of α-K6P2W18O62 · 14H2O. This pilot study provided evidence that α-K6P2W18O62 · 14H2O and oxacillin can overcome resistance in ATCC 43300.
- Received July 24, 2018.
- Accepted September 8, 2018.
American Society for Clinical Laboratory Science